Abstract
Pyrazinamide (PZA) is a first line anti-tubercular drug for which the mechanism of action remains unresolved. Recently, it was proposed that the active form of PZA, pyrazinoic acid (POA), disrupts the ribosome rescue process of trans-translation in Mycobacterium tuberculosis. This model suggested that POA binds within the carboxy-terminal domain of ribosomal protein S1 (RpsA) and inhibits trans-translation leading to accumulation of stalled ribosomes. Here, we demonstrate that M. tuberculosis RpsA interacts with single stranded RNA, but not with POA. Further, we show that an rpsA polymorphism previously identified in a PZA resistant strain does not confer PZA resistance when reconstructed in a laboratory strain. Finally, by utilizing an in vitro trans-translation assay with purified M. tuberculosis ribosomes we find that an interfering oligonucleotide can inhibit trans-translation, yet POA does not inhibit trans-translation. Based on these findings, we conclude that the action of PZA is entirely independent of RpsA and trans-translation in M. tuberculosis.
Introduction
Pyrazinamide (PZA) is a first line anti-tubercular drug that has enabled a reduction in tuberculosis (TB) treatment duration from 9 to 6 months and has played a critical role in lowering relapse rates1, 2. Efficacy of PZA is dependent on hydrolysis to pyrazinoic acid (POA) by the Mycobacterium tuberculosis encoded pyrazinamidase/nicotinamidase PncA3. As PncA is non-essential for growth and pathogenesis of M. tuberculosis, the majority of PZA resistant clinical isolates harbor loss-of-function mutations in pncA 4. While PZA shows sterilizing activity against M. tuberculosis in humans and in animal models of TB infection5–7, it shows no notable effect on growth of M. tuberculosis in standard laboratory culture medium8. Since stresses such as acidic pH9, nutrient limitation10 and anaerobiosis11 potentiate the anti-tubercular action of PZA, it has been suggested that this drug selectively targets slow growing and non-growing populations of M. tuberculosis 12. However, it is important to note that despite this condition-dependence for drug action, PZA and POA do show inhibitory activity against actively replicating M. tuberculosis 10, 13, indicating that the function(s) disrupted by POA is critical for fitness of the bacilli regardless of their growth status. Understanding the mechanistic basis for action of this important drug will guide discovery efforts for next generation compounds that show improved activity and can circumvent emerging resistance to existing drugs.
Several models have been proposed to explain the anti-tubercular action of PZA. Based on the observation that the action of POA, a weak acid, is enhanced by incubation of mycobacteria under acidic conditions, it was proposed that this drug might act as a proton ionophore causing collapse the cellular membrane potential and intrabacterial acidification14. However, since acidic pH is not strictly required for the action of PZA and POA, and treatment with these drugs does not result in rapid disruption of membrane potential nor intrabacterial acidification, ionophoric activity of POA seems insufficient to explain its anti-tubercular action10, 15. Another study involving the PZA analog 5-Cl-PZA led to the proposal that POA directly inhibits fatty acid synthase I (FAS-I)16. However, while fatty acid synthesis is inhibited following treatment of mycobacteria with PZA and POA, the POA IC50 for FAS-I is orders of magnitude greater than that of 5-Cl-PZA17. Since PZA and 5-Cl-PZA show similar inhibitory concentrations for susceptible strains of M. tuberculosis 18, it is reasonable to predict that inhibition of fatty acid synthesis is a downstream consequence of PZA action. Recent molecular studies have established a connection between PZA action and coenzyme A (CoA) metabolism19–24. These studies have shown that PZA action can be antagonized by exogenously supplied intermediates of the CoA biosynthetic pathway and that treatment of bacilli with PZA and POA leads to a reduction in CoA abundance19, 21–23. Mutations in the carboxy-terminal domain of panD, encoding L-aspartate decarboxylase of the CoA biosynthetic pathway, are associated with PZA resistance in broth culture and in the murine model of infection20, 21, 24 leading to the suggestion that PanD might be a target of POA. However, a M. tuberculosis strain deleted for panD still shows conditional susceptibility to PZA19, demonstrating that PanD cannot be the exclusive target of this drug. Since CoA is a critical cofactor in fatty acid synthesis, it is likely that disruption of CoA homeostasis explains the ability of PZA to interfere with this pathway. Additional studies are required to delineate the connection between CoA metabolism and PZA action.
In addition to the models described above, it has also been suggested that POA might act by inhibiting trans-translation25, a process used by bacteria to liberate ribosomes that have stalled on mRNA transcripts lacking an in-frame stop codon26. This pathway requires small protein B (SmpB) for recruitment of the dual function transfer-messenger RNA (tmRNA) that promotes release of the stalled ribosome from its non-stop mRNA with subsequent tagging and release of the incomplete nascent peptide26. Evidence for POA-mediated inhibition of trans-translation in M. tuberculosis included 1) an apparent interaction between POA and ribosomal protein S1 (RpsA) as demonstrated through the use of isothermal titration calorimetry (ITC), 2) the PZA resistant clinical isolate DHMH444 (PZA MIC of 300 µg/ml) was found to have a polymorphism in rpsA (rpsAΔA438; deletion of alanine codon 438), 3) five-fold over-expression of rpsA in M. tuberculosis strain H37Ra allegedly conferred resistance to PZA (MIC of 500 µg/ml), and 4) POA was said to inhibit trans-translation in a cell-free assay25. As trans-translation is important for growth and stress tolerance of many bacteria27–29, this model seemed consistent with the ability of PZA to target growing and non-growing populations of M. tuberculosis.
Despite its plausibility, this model is inconsistent with previous reports of the role of RpsA in trans-translation and the PZA and POA susceptibility phenotypes of M. tuberculosis strain DHMH444. While RpsA has been shown to interact with tmRNA, RpsA is not actually required for the trans-translation pathway in bacterial systems in which this has been evaluated, such as Escherichia coli 30 and Thermus thermophilus 31, 32. Further, a strain of M. tuberculosis altered for trans-translation showed enhanced susceptibility to antibiotics that interfere with translation, yet, displayed no difference in PZA susceptibility relative to the parental control33. In addition, it has been shown that M. tuberculosis strain DHMH444 bearing the rpsA∆A438 polymorphism is susceptible to PZA in a murine model of infection34. While this clinical isolate shows low level resistance to PZA, the role of the rpsA∆A438 polymorphism has not been evaluated through the use of matched isogenic strains. This is an important consideration since clinical isolates of M. tuberculosis can show over 1,000 genetic polymorphisms35. Importantly, strain DHMH444 is known to be fully susceptible to POA in broth culture, and the moderate in vitro PZA resistance of this strain has previously been attributed to its reduced PncA activity36. Collectively, these observations undermine the likelihood of a role for RpsA and trans-translation in PZA action. To resolve these inconsistencies, we further examined the role of RpsA in PZA susceptibility of M. tuberculosis and biochemically evaluated the interaction of POA with RpsA and the trans-translation complex. Herein we present evidence that 1) the rpsA∆A438 mutation is not associated with PZA resistance, 2) overexpression of rpsA does not confer PZA resistance, 3) RpsA does not interact with POA, and 4) POA does not inhibit trans-translation. Based on our findings and previously published observations, we conclude that PZA action is entirely independent of RpsA and trans-translation in M. tuberculosis.
Results and Discussion
Using isogenic M. tuberculosis laboratory strains we assessed the impact of the rpsA∆A438 polymorphism on PZA susceptibility. Utilizing specialized linkage transduction37 we reconstructed the rpsA∆A438 polymorphism in the PZA susceptible M. tuberculosis laboratory strain H37Ra. Three independent rpsA∆A438 and two matched wild type strains were verified by amplifying and sequencing the rpsA locus, followed by full genome resequencing. The PZA minimum inhibitory concentration (MIC) for these strains was found to be indistinguishable from that of the parental strain (Fig. 1A). Thus, we conclude that the rpsA∆A438 polymorphism is not linked with PZA susceptibility. This observation is not surprising given that the borderline PZA resistant M. tuberculosis strain DHMH444 maintains full susceptibility to POA in broth culture36 and PZA susceptibility in a murine model of tuberculosis infection34. It has been suggested that the low level resistance of this clinical isolate is likely due to its reduced PncA activity36. Given the large number of polymorphisms between M. tuberculosis clinical isolates and common laboratory strains35, it is likely that strain DHMH444 has additional unidentified mutations that are responsible for its low level PZA resistance phenotype.
Figure 1.
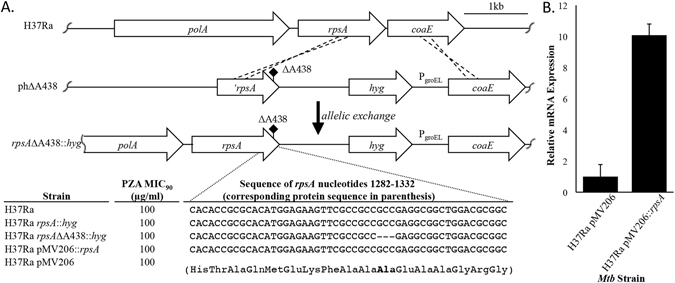
The rpsA∆A438 polymorphism and rpsA over-expression do not confer PZA resistance in M. tuberculosis. (A) Schematic representation of specialized linkage transduction used to introduce the rpsA∆A438 mutation and corresponding nucleotide sequences of the rpsA locus of respective strains. Wild type and rpsA∆A438 mutant strains were tested for the minimum concentration of PZA that was required to inhibit at least 90% of growth relative to the no drug control (MIC90) over 2 weeks of incubation in supplemented 7H9 medium (pH 5.8). (B) Over-expression of rpsA in M. tuberculosis. qRT-PCR was performed on RNA extracted from M. tuberculosis strains H37Ra and H37Ra pMV206::rpsA. Relative mRNA expression was calculated using sigA transcripts for normalization. All MIC90 determinations (A) and qRT-PCR assays (B) were conducted with at least three independent replicates. Three independent isolates bearing the rpsA∆A438 polymorphism, confirmed by full genome sequencing, were assessed. Error bars represent standard deviation from the mean of three independent biological replicates.
To further evaluate the connection between RpsA and PZA resistance, rpsA was over-expressed in the laboratory strain M. tuberculosis H37Ra using an analogous mycobacterial expression vector as previously described25. While the previous report did not show data for over-expression of rpsA 25, quantitative real time PCR (qRT-PCR) from the M. tuberculosis strain used herein confirmed 10-fold over-expression of rpsA (Fig. 1B). In contrast to the previous report25, over-expression of rpsA did not confer PZA resistance (Fig. 1A). The basis for this discrepancy in PZA susceptibility with rpsA over-expression is unclear, yet, we conclude that PZA susceptibility of M. tuberculosis is not affected by abundant over-expression of rpsA.
To evaluate whether PZA treatment influences rpsA expression levels in M. tuberculosis, we reanalyzed transcriptional array data from Boshoff et al.38. In this previous study, M. tuberculosis cultures were treated with a multitude of different anti-tubercular agents and transcriptional microarrays were performed to gain insight on drug action38. We re-evaluated data sets from treatment of M. tuberculosis with PZA at 1x and 10x the MIC over a time course of 16 hours and found rpsA expression levels were relatively unchanged relative to the no-drug control (Supplementary Fig. S1). Thus, PZA treatment does not appear to impact expression of this purported target.
Next, we utilized isothermal titration calorimetry (ITC) to re-examine the purported interaction between M. tuberculosis RpsA and POA. During protein synthesis, RpsA aids in translation initiation via direct binding of single stranded mRNA39. E. coli RpsA has been shown to have two RNA binding sites with different affinities for polyC RNA40–42. Utilizing purified recombinant M. tuberculosis RpsA prepared as described in25 and polyC RNA as a ligand, we observed a bimodal association curve consistent with the presence of two high-affinity RNA binding sites on RpsA (Fig. 2A). Fitting the data to a multi-binding site model we determined that the higher affinity site of M. tuberculosis RpsA bound polyC RNA with a Ka of 8.91 × 107 ± 5.42 × 107 M−1 while the lower affinity site bound polyC RNA with a Ka of 8.48 × 106 ± 1.85 × 106 M−1 (Fig. 2A). These data demonstrate that the purified RpsA is properly folded and has the expected biochemical properties. Despite this robust interaction with polyC RNA and in contrast to that which was described previously25, no signal was observed when POA was titrated with RpsA (Fig. 2B). These data demonstrate that M. tuberculosis RpsA and POA do not show detectable interaction.
Figure 2.
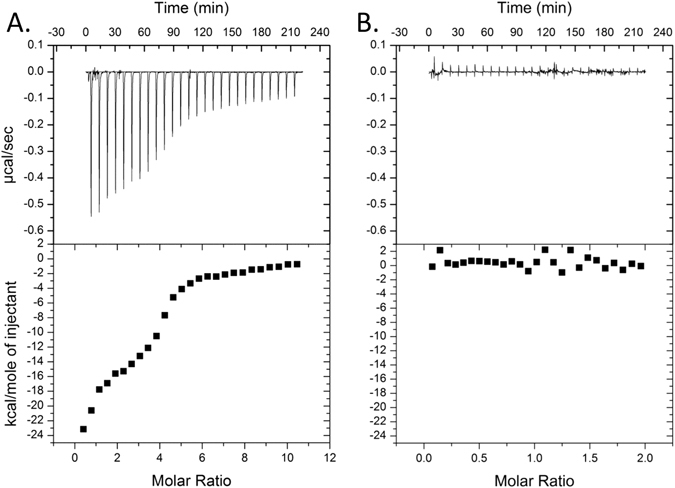
M. tuberculosis RpsA interacts with polyC RNA, but not with POA. Isothermal titration calorimetry was used to assess the binding between RpsA and either polyC RNA (A) or POA (B). In each plot, the top panel represents the heat produced per injection as µcal/sec while the bottom panel shows the change in enthalpy (kcal/mole) as a function of the molar ratio of the two ligands. Titrations were performed at 25 °C using 10 mM phosphate buffer (pH 7.4). POA and polyC RNA solutions were adjusted to pH 7.4.
ITC is a sensitive approach for measurement of thermodynamic reactions in solution. Injection of a weak acid, such as POA (pKa of 2.9), into a modestly buffered solution can generate a robust signal due to pH dependent proton dissociation. In the previous study, a saturated solution of POA (~70 mM) was used for evaluating interaction with recombinant M. tuberculosis RpsA, and was erroneously compared to titrations involving 100 µM POA with recombinant RpsA from either M. tuberculosis strain DHMH444 or M. smegmatis 25. As anticipated, we find that when a saturated POA solution is titrated into 10 mM phosphate buffer, a robust signal due to proton dissociation is observed and is entirely independent of the presence of RpsA (Fig. 3A). This signal is fully abrogated when the pH of the saturated POA solution is adjusted to that of the diluent buffer (Fig. 3B), confirming that this robust signal is the result of acid dissociation. As the authors of the previous report describe using saturating levels of POA when signal was observed and ~700 fold less POA when signal was not observed, we speculate that the reported signal for M. tuberculosis RpsA and POA was not due to interaction between these ligands, but was instead due to heat of proton dissociation of the weak acid POA.
Figure 3.
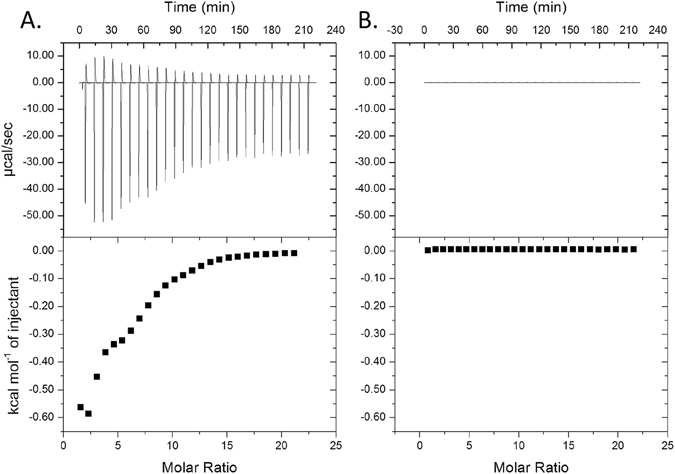
Unbuffered POA gives a robust ITC signal when titrated into neutral buffer. Saturated POA ((A) unadjusted pH 2.3; (B) pH adjusted to 7.4) was injected into 10 mM phosphate buffer (pH 7.4).
These ligand interaction studies call into question the initial basis for identification of RpsA as an interaction partner of POA. In the previous study25, the authors utilized a column in which 5-hydroxy-POA was covalently coupled to a Sepharose resin to purify proteins from a cellular lysate of M. tuberculosis strain H37Ra. Rather than utilizing free POA to elute proteins that bound specifically to the POA moiety, bound proteins were stripped from the column with 25% ethylene glycol. Through this approach, several proteins were obtained, including Rv2783c, RpsA, Rv2731, Rv3169, and others that were not specifically identified. As RpsA is one of the most abundant proteins in the M. tuberculosis proteome43 and this purification approach resulted in isolation of numerous proteins, we speculate that identification of RpsA was merely due to its high abundance in the cellular extracts that were used.
To specifically address whether POA can inhibit the tagging activity mediated by the trans-translation pathway, we utilized an in vitro trans-translation assay44. A gene encoding dihydrofolate reductase (DHFR) lacking a stop codon (DHFR-NS) was used as a template in a transcription/translation assay containing M. tuberculosis ribosomes, tmRNA and SmpB. Expression of DHFR-NS with M. tuberculosis components resulted in tagging of the DHFR-NS demonstrating that trans-translation functions in vitro with M. tuberculosis components (Fig. 4). Incubation with 1 mM POA resulted in no substantial reduction in the tagging activity of trans-translation (1.6 ± 4%), while incubation with an anti-sense SsrA oligonucleotide exhibited >90% reduction in tagging activity (Fig. 4). These observations lead us to conclude that POA does not inhibit trans-translation in M. tuberculosis.
Figure 4.
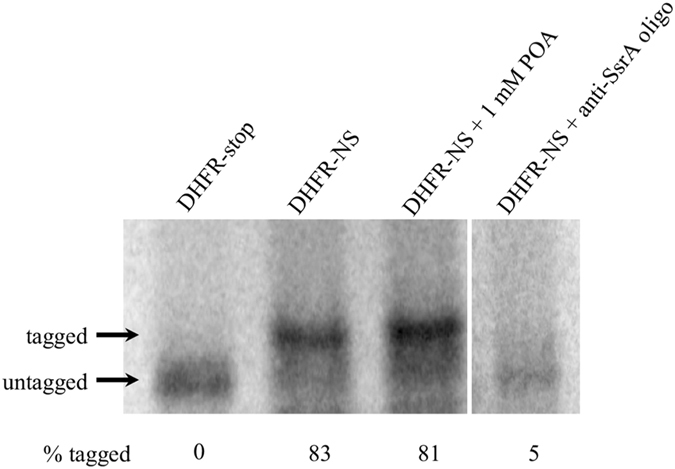
POA does not inhibit trans-translation in vitro. trans-Translation reactions with 50 nM M. tuberculosis ribosomes, 150 nM M. tuberculosis tmRNA, 150 nM M. tuberculosis SmpB, and 640 nM template DNA. Where indicated pyrazinoic acid (Sigma) was added to 1 mM or anti-SsrA oligonucleotide was added to 2 µM. Reactions were incubated at 37 °C 3 h and analyzed by SDS-PAGE followed by phosporimaging. This image was cropped to improve clarity. A full-length image is provided as Supplementary Figure S2.
These results conflict with those described in Shi et al.25. There are several important differences between the assay conditions that likely account for the discrepant observations. First, the previous study used a DHFR gene that was followed by 26 additional codons (8 rare AGG codons and 18 additional codons) and a stop codon as the template for expression. Although expression of genes containing rare codons can lead to trans-translation in E. coli cells, the mRNA must first be cut by a nuclease before trans-translation can occur45. No RNases were included in the reactions described in25, so expression of the gene used in those reactions would not induce a high rate of trans-translation and instead would lead to production of DHFR containing the additional 26 template encoded amino acids. Second, in Shi et al. 25 pre-tmRNA was added to reactions mixtures and did not contain the enzymes required for pre-tmRNA processing and maturation46–49. Pre-tmRNA cannot be charged with alanine and is inactive for trans-translation49. Despite using inactive tmRNA and a gene that would not result in a high rate of trans-translation, Shi et al.25 report 100% tagged protein in the absence of POA, and observe complete inhibition of protein synthesis with ≥25 µg/ml POA using their DHFR-8xAGG gene. It is important to note that by the nature of this assay, inhibition of trans-translation would still result in production of a band corresponding to untagged DHFR as 35S-methionine is incorporated during translation of the nascent peptide regardless of subsequent engagement of trans-translation50. In other words, translation is a prerequisite for trans-translation and failure to observe untagged protein is indicative of inhibition of translation. However, failure to observe signal could also be explained by failure to load sample in the respective lanes. Regardless, the most likely explanation for the observations in the previous study is that the authors did not correctly assign the DHFR bands in their figure and no trans-translation occurred in their assays. In contrast, in our study, the assays shown in Fig. 4 use mature tmRNA and a template gene with no stop codon, which has been shown to induce trans-translation44. The anti-SsrA oligo control in Fig. 4 demonstrates that the change in mobility of the protein product we observed is due to trans-translation.
Collectively, our observations demonstrate that RpsA and trans-translation do not have a role in the mode of action of PZA and that the mechanistic basis for PZA susceptibility remains to be elucidated. Given the recent observations by multiple independent laboratories that PZA action can be antagonized by intermediates of the CoA biosynthetic pathway and that alterations in CoA metabolism influence PZA susceptibility19–24, it is likely that this drug directly impairs a critical player in CoA metabolism. Interestingly, mutations in the gene for the ClpC1 unfoldase have also recently been recognized in M. tuberculosis PZA resistant laboratory isolates24, 51, 52, yet, the mechanistic basis for the association between ClpC1 and PZA resistance remain unclear. Considering the unparalleled in vivo sterilizing activity of PZA and the increasing global burden of multidrug-resistant and extensively drug-resistant tuberculosis, elucidating the requirements for susceptibility of M. tuberculosis to key drugs such as PZA is of paramount importance for the optimization of impactful treatment regimens.
Materials and Methods
Bacterial strains and growth conditions
Mycobacterium tuberculosis strain H37Ra was grown in Middlebrook 7H9 medium (Difco) supplemented with 10% (vol/vol) oleic acid-albumin-dextrose-catalase (OADC) (Difco), 0.2% (vol/vol) glycerol, and 0.05% (vol/vol) tyloxapol. Escherichia coli strains DH5α used for the propagation of phasmids and plasmids and BL21(DE3) used for overexpression and purification of protein were grown in Lysogeny Broth (LB). Antibiotics hygromycin (150 μg ml−1) and kanamycin (50 μg ml−1) were used as necessary.
RpsA Expression and Purification
For RpsA expression and purification, M. tuberculosis encoded rpsA was amplified by PCR, digested with BamHI and HindIII, and ligated into pET-28b+ digested with the same enzymes. The recombinant plasmid was transformed and propagated in BL21(DE3) selected with kanamycin. Growing BL21(DE3) containing pET-28b+rpsA was induced with 0.25 mM isopropyl β-D-1-thiogalactopyranoside (IPTG) for 3 hours at 37 °C. Induced cells were harvested via centrifugation and resuspended in 50 mM NaH2PO4, 300 mM NaCl, 10 mM imidazole pH 8.0. Cells were disrupted by sonication and debris pelleted via centrifugation. Supernatant was bound to Ni2+-NTA resin (Qiagen), previously washed and equilibrated with 50 mM NaH2PO4, 300 mM NaCl, 20 mM imidazole pH 8.0, by mixing at 4 °C. Resin supernatant slurry was packed into a chromatography column and washed with 20 column volumes of 50 mM NaH2PO4, 300 mM NaCl, 20 mM imidazole pH 8.0. Bound RpsA was eluted with 5 column volumes of 50 mM NaH2PO4, 300 mM NaCl, 250 mM imidazole pH 8.0. Eluted RpsA was dialyzed with 800 ml 10 mM phosphate buffer twice for 3 hours and once for 16 hours. Overexpression and purification were confirmed via SDS-PAGE gel.
Isothermal titration calorimetry (ITC) assay
The ITC interaction assays were conducted on the MicroCal VP-ITC at 25.0 °C. POA, RpsA, and the polyC-RNA positive control were dissolved in 10 mM phosphate buffer (pH 7.4). The pH of the POA buffer solution was adjusted to pH 7.4 to account for any pH change due to POA, unless otherwise indicated. The drugs were loaded into the syringe at the indicated concentrations. Each experiment consisted of 26 10 μl injections over a 2 second duration into 1449.7 μl of ligand within the cell. The solution mixtures were stirred at 300 rpm and the interval between injections was set at 500 seconds. Origin 7 software (Origin®) was used to collect and analyze the data.
qRT-PCR
For quantification of rpsA overexpression quantitative reverse transcription-PCR (qRT-PCR) was performed. Briefly, mid exponential phase M. tuberculosis was harvested via centrifugation. Cell pellet was resuspended in 100 μl 10 mM Tris-HCl, 1 mM EDTA, 15 mg/ml lysozyme and incubated at 37 °C for 16 hours. RNA was extracted using the E.Z.N.A.TM bacterial RNA kit (Omega Biotek). Remaining DNA was removed by treatment with TURBO DNA-freeTM kit (Ambion). Gene specific primers for qRT-PCR were designed with Primer3 software. qRT-PCR was performed with the QuantiFast® SYBR® Green RT-PCR kit (Qiagen). qRT-PCR reactions were prepared with 2X QuantiFast SYBR Green RT-PCR master mix, 10 μM primers, 0.1 μl QuantiFast RT Mix, 1 ng RNA and were run on a LightCycler®480 with following cycle conditions: 50 °C for 10 min, 95 °C for 5 min, 35 cycles of 95 °C for 10 s, 60 °C for 10 s, and 72 °C for 20 s with fluorescence quantification each cycle. Melting curve cycle of 95 °C for 15 s, 60 °C for 15 s, and 95 °C with 2% ramp rate to determine product specificity. qRT-PCR reactions lacking reverse transcriptase were performed to test for contaminating DNA.
Cloning of allelic exchange and overexpression strains
To generate the rpsA ∆A438 polymorphism in M. tuberculosis rpsA was first cloned into p0004s using PCR amplification followed by a 4 piece ligation. The 5′ piece was amplified with the primers rpsA_5′_F and rpsA_5′_R and digested with PacI and NheI. The 3′ piece was then amplified via PCR with the primers rpsA_3′_F and rpsA_3′_R and digested with NcoI and NdeI. The primers designed for the rpsA∆A438 quick change, rpsA∆A438_F and rpsA∆A438_R, were then used to introduce the alanine deletion in rpsA via PCR amplification. The resulting cosmid was then inserted into phAE159 and was introduced into M. tuberculosis via specialized transduction.
For RpsA overexpression, M. tuberculosis encoded rpsA was amplified by PCR with primers indicated in Table S1, digested with NheI and XbaI, and ligated into mycobacterial replicative expression vector pMV261 digested with XbaI. The recombinant plasmid was transformed and propagated in DH5α selected with kanamycin. For RpsA overexpression H37Ra was electroporated with the rpsA overexpression plasmid and selected on supplemented 7H10 containing kanamycin.
Pyrazinamide susceptibility testing
Antimicrobial susceptibility was determined by measuring optical density of respective cultures at 600 nm (OD600). PZA susceptibility testing was performed using supplemented 7H9 medium adjusted to pH 5.8. The minimum inhibitory concentration (MIC90) for antimicrobial compounds was defined as the minimum concentration required for inhibition of at least 90% of growth, relative to the no antimicrobial control. Growing M. tuberculosis H37Ra was diluted to an OD600 of 0.01 in 5 ml of supplemented 7H9 medium in 30 ml square Nalgene bottles. Antimicrobial compounds were added to the final concentrations indicated in the text. Cultures were incubated at 37 °C with shaking on a rotary platform at 100 rpm for 14 days. All results presented are from a minimum of three independent determinations.
Ribosome purification
Cells were grown in supplemented 7H9 until mid exponential phase (OD600 of 0.5) and subjected to French pressure lysis. The lysate was cleared by centrifugation at 30,000 × g for 20 min, and crude ribosomes were harvested from the supernatant by centrifugation at 100,000 × g for 2 h. The pellet was washed 3 X in Buffer II (20 mM Tris-HCl [pH 7.6], 1 M ammonium acetate, 10 mM magnesium acetate, 6 mM ß-ME), and resuspended in Buffer I (10 mM Tris-HCl [pH 7.6], 100 mM ammonium acetate, 10 mM magnesium acetate, 6 mM β-ME). 70S ribosomes were isolated by sucrose density fractionation (10–40% sucrose in 10 mM Tris-HCl [pH 8.0], 30 mM KCl, 10 mM magnesium acetate).
Isolation of M. tuberculosis tmRNA and SmpB
M. tuberculosis ssrA was amplified by PCR using primers MtbSsrAF and MtbSsrAR to place it under control of a T7 promoter. The product was gel purified and used as template in a second PCR reaction with primers MtbSsrAF and MtbSsrAR. The product was transcribed in vitro and purified as described previously for E. coli tmRNA44. M. tuberculosis smpB was amplified by PCR using primers TB_SmpB_F and TB_SmpB_R, digested with HindIII and NdeI, and ligated into pET28b that had been digested with the same enzymes. SmpB was produced and purified as described previously for E. coli SmpB44.
In vitro trans-translation assays
Template construction and reaction conditions were as previously described44, with the following modifications. DHFR-stop and DHFR-NS templates were made by PCR using T7 universal primer and either Stop_UTR_DHFR_FL or NS_UTR_DHFR_FL. trans-translation reactions used the PURExpress ribosome-free kit (New England Biolabs) with 50 nM M. tuberculosis ribosomes, 150 nM M. tuberculosis tmRNA, 150 nM M. tuberculosis SmpB, and 640 nM template DNA. Where applicable, pyrazinoic acid (Sigma) was added to 1 mM or anti-SsrA oligo was added to 2 µM. Reactions were incubated at 37 °C 3 h and analyzed by SDS-PAGE followed by phosporimaging44.
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Electronic supplementary material
Acknowledgements
This work was supported by grants from the NIH (AI123146) and Bill & Melinda Gates Foundation (Grand Challenges Explorations), and institutional startup funds from the University of Minnesota to ADB. K.C.K. and H.A.F. were supported by grant GM068720 from NIH. N.A.D was supported by an institutional training grant from the NIH (HL007741). We thank Dr. William R. Jacobs, Jr. for providing M. tuberculosis strains H37Ra and H37Rv, Dr. Yusuke Minato for assistance in analyzing mutant strains of M. tuberculosis, Joshua Thiede for help with analyzing transcriptional data, and Chris Rae who helped provide reagents for in vitro trans-translation assays.
Author Contributions
N.A.D., N.D.P. and A.D.B. designed and performed experiments involving construction and characterization of recombinant bacterial strains. N.A.D. performed experiments involving use of isothermal titration calorimetry. H.A.F. and K.C.K. designed and performed in vitro trans-translation experiments. All authors contributed to writing and editing of the manuscript.
Competing Interests
The authors declare that they have no competing interests.
Footnotes
Nicholas A. Dillon and Nicholas D. Peterson contributed equally to this work.
Electronic supplementary material
Supplementary information accompanies this paper at doi:10.1038/s41598-017-06415-5
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Somner A, Angel J. A controlled trial of six months chemotherapy in pulmonary tuberculosis. First Report: results during chemotherapy. Br J Dis Chest. 1981;75:141–153. doi: 10.1016/0007-0971(81)90046-2. [DOI] [PubMed] [Google Scholar]
- 2.East African/British Medical Research Councils. Controlled Clinical Trial of Four Short-Course (6-Month) Regimens of Pulmonary Tuberculosis: Third Report. Lancet304, 237–240 (1974). [PubMed]
- 3.Scorpio A, Zhang Y. Mutations in pncA, a gene encoding pyrazinamidase/nicotinamidase, cause resistance to the antituberculous drug pyrazinamide in tubercle bacillus. Nat Med. 1996;2:662–667. doi: 10.1038/nm0696-662. [DOI] [PubMed] [Google Scholar]
- 4.Stoffels K, Mathys V, Fauville-Dufaux M, Wintjens R, Bifani P. Systematic Analysis of Pyrazinamide-Resistant Spontaneous Mutants and Clinical Isolates of Mycobacterium tuberculosis. Antimicrob Agents Chemother. 2012;56:5186–5193. doi: 10.1128/AAC.05385-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dessau, F. I., Yeager, R. L., Burger, F. J. & Williams, J. H. Pyrazinamide (aldinamide) in experimental tuberculosis of the guinea pig. Am Rev Tuberc65, 519–522 (1952). [PubMed]
- 6.Malone LSA, Lindh H, McKenzie D, Kiser JS, Williams JH. The effect of pyrazinamide (aldinamide) on experimental tuberculosis in mice. Am Rev Tuberc. 1952;65:511–518. [PubMed] [Google Scholar]
- 7.Yeager RL, Munroe WG, Dessau FI. Pyrazinamide (aldinamide) in the treatment of pulmonary tuberculosis. Am Rev Tuberc. 1952;65:523–546. [PubMed] [Google Scholar]
- 8.Tarshis M, Weed WJ. Lack of significant in vitro sensitivity of Mycobacterium tuberculosis to pyrazinamide on three different solid media. Am Rev Tuberc. 1953;67:391–395. doi: 10.1164/art.1953.67.3.391. [DOI] [PubMed] [Google Scholar]
- 9.McDermott W, Tompsett R. Activation of pyrazinamide and nicotinamide in acidic environments in vitro. Am Rev Tuberc. 1954;70:748–754. doi: 10.1164/art.1954.70.4.748. [DOI] [PubMed] [Google Scholar]
- 10.Peterson ND, Rosen BC, Dillon NA, Baughn AD. Uncoupling Environmental pH and Intrabacterial Acidification from Pyrazinamide Susceptibility in Mycobacterium tuberculosis. Antimicrob Agents Chemother. 2015;59:7320–7326. doi: 10.1128/AAC.00967-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wade MM, Zhang Y. Anaerobic incubation conditions enhance pyrazinamide activity against Mycobacterium tuberculosis. J Med Microbiol. 2004;53:769–773. doi: 10.1099/jmm.0.45639-0. [DOI] [PubMed] [Google Scholar]
- 12.Zhang Y, Mitchison D. The curious characteristics of pyrazinamide: a review. Int J Tuberc Lung Dis. 2003;7:6–21. [PubMed] [Google Scholar]
- 13.Via LE, et al. Host-Mediated Bioactivation of Pyrazinamide: Implications for Efficacy, Resistance, and Therapeutic Alternatives. ACS Infect Dis. 2015;1:203–214. doi: 10.1021/id500028m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang Y, Wade MM, Scorpio A, Zhang H, Sun Z. Mode of action of pyrazinamide: disruption of Mycobacterium tuberculosis membrane transport and energetics by pyrazinoic acid. J Antimicrob Chemother. 2003;52:790–795. doi: 10.1093/jac/dkg446. [DOI] [PubMed] [Google Scholar]
- 15.Baughn AD, et al. Mutually Exclusive Genotypes for Pyrazinamide and 5-Chloropyrazinamide Resistance Reveal a Potential Resistance-Proofing Strategy. Antimicrob Agents Chemother. 2010;54:5323–5328. doi: 10.1128/AAC.00529-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zimhony O, Cox JS, Welch JT, Vilchèze C, Jacobs WRJ. Pyrazinamide inhibits the eukaryotic-like fatty acid synthetase I (FASI) of Mycobacterium tuberculosis. Nat Med. 2000;6:1043–1047. doi: 10.1038/79558. [DOI] [PubMed] [Google Scholar]
- 17.Boshoff HI, Mizrahi V, Barry CE. III Effects of pyrazinamide on fatty acid synthesis by whole Mycobacterial cells and purified Fatty Acid Synthase I. J Bacteriol. 2002;184:2167–2172. doi: 10.1128/JB.184.8.2167-2172.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cynamon MH, Speirs RJ, Welch JT. In Vitro Antimycobacterial Activity of 5-Chloropyrazinamide. Antimicrob Agents Chemother. 1998;42:462–463. doi: 10.1128/aac.42.2.462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dillon NA, Peterson ND, Rosen BC, Baughn AD. Pantothenate and Pantetheine Antagonize the Antitubercular Activity of Pyrazinamide. Antimicrob Agents Chemother. 2014;58:7258–7263. doi: 10.1128/AAC.04028-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shi W, et al. Aspartate decarboxylase (PanD) as a new target of pyrazinamide in Mycobacterium tuberculosis. Emerg Microbes Infect. 2014;3:e58. doi: 10.1038/emi.2014.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gopal P, et al. Pyrazinamide resistance is caused by two distinct mechanisms: prevention of coenzyme A depletion and loss of virulence factor synthesis. ACS Infect Dis. 2016;2:616–626. doi: 10.1021/acsinfecdis.6b00070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang S, et al. Mutations in panD encoding aspartate decarboxylase are associated with pyrazinamide resistance in Mycobacterium tuberculosis. Emerg Microbes Infect. 2013;2:e34. doi: 10.1038/emi.2013.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rosen BC, Dillon NA, Peterson ND, Minato Y, Baughn AD. Long-Chain Fatty Acyl-CoA Ligase FadD2 Mediates Intrinsic Pyrazinamide Resistance in Mycobacterium tuberculosis. Antimicrob Agents Chemother. 2017;61:e02130–02116. doi: 10.1128/AAC.02130-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gopal, P. et al. In Vivo-Selected Pyrazinoic Acid-Resistant Mycobacterium tuberculosis Strains Harbor Missense Mutations in the Aspartate Decarboxylase PanD and the Unfoldase ClpC1. ACS Infectious Diseases doi:10.1021/acsinfecdis.7b00017 (2017). [DOI] [PMC free article] [PubMed]
- 25.Shi W, et al. Pyrazinamide Inhibits trans-Translation in Mycobacterium tuberculosis. Science. 2011;333:1630–1632. doi: 10.1126/science.1208813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Keiler KC, Feaga HA. Resolving Nonstop Translation Complexes Is a Matter of Life or Death. J Bacteriol. 2014;196:2123–2130. doi: 10.1128/JB.01490-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Luidalepp, H., Hallier, M. & Felden, B. tmRNA Decreases the bactericidal activity of aminoglycosides and the susceptibility to inhibitors of cell wall synthesis. RNA Biol2, 70–74 (2005). [DOI] [PubMed]
- 28.Thibonnier M, Thiberge JM, De Reuse H. trans-Translation in Helicobacter pylori: essentiality of ribosome rescue and requirement of protein tagging for stress resistance and competence. PLoS ONE. 2008;3:e3810. doi: 10.1371/journal.pone.0003810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Svetlanov A, Puri N, Mena P, Koller A, Wali Karzai A. Francisella tularensis tmRNA system mutants are vulnerable to stress, avirulent in mice, and provide effective immune protection. Mol Microbiol. 2012;85:122–141. doi: 10.1111/j.1365-2958.2012.08093.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McGinness KE, Sauer RT. Ribosomal protein S1 binds mRNA and tmRNA similarly but plays distinct roles in translation of these molecules. Proc Natl Acad Sci USA. 2004;101:13454–13459. doi: 10.1073/pnas.0405521101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Takada K, et al. Thermus thermophilus tmRNA and trans-translation. Nucleic Acids Symp Ser. 2007;51:369–370. doi: 10.1093/nass/nrm185. [DOI] [PubMed] [Google Scholar]
- 32.Qi H, Shimizu Y, Ueda T. Ribosomal Protein S1 Is not Essential for the trans-Translation Machinery. J Mol Biol. 2007;368:845–852. doi: 10.1016/j.jmb.2007.02.068. [DOI] [PubMed] [Google Scholar]
- 33.Personne Y, Parish T. Mycobacterium tuberculosis possesses an unusual tmRNA rescue system. Tuberculosis. 2014;94:34–42. doi: 10.1016/j.tube.2013.09.007. [DOI] [PubMed] [Google Scholar]
- 34.Klemens SP, Sharpe CA, Cynamon MH. Activity of pyrazinamide in a murine model against Mycobacterium tuberculosis isolates with various levels of in vitro susceptibility. Antimicrob Agents Chemother. 1996;40:14–16. doi: 10.1128/aac.40.1.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fleischmann RD, et al. Whole-Genome Comparison of Mycobacterium tuberculosis Clinical and Laboratory Strains. J Bacteriol. 2002;184:5479–5490. doi: 10.1128/JB.184.19.5479-5490.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Speirs RJ, Welch JT, Cynamon MH. Activity of n-propyl pyrazinoate against pyrazinamide-resistant Mycobacterium tuberculosis: investigations into mechanism of action of and mechanism of resistance to pyrazinamide. Antimicrob Agents Chemother. 1995;39:1269–1271. doi: 10.1128/AAC.39.6.1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vilcheze C, et al. Transfer of a point mutation in Mycobacterium tuberculosis inhA resolves the target of isoniazid. Nat Med. 2006;12:1027–1029. doi: 10.1038/nm1466. [DOI] [PubMed] [Google Scholar]
- 38.Boshoff HIM, et al. The Transcriptional Responses of Mycobacterium tuberculosis to Inhibitors of Metabolism: Novel insights into drug mechanisms of action. J Biol Chem. 2004;279:40174–40184. doi: 10.1074/jbc.M406796200. [DOI] [PubMed] [Google Scholar]
- 39.Qu X, Lancaster L, Noller HF, Bustamante C, Tinoco I. Ribosomal protein S1 unwinds double-stranded RNA in multiple steps. Proc Natl Acad Sci USA. 2012;109:14458–14463. doi: 10.1073/pnas.1208950109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Draper DE, von Hippel PH. Nucleic acid binding properties of Escherichia coli ribosomal protein S1. I. Structure and interactions of binding site I. J Mol Biol. 1978;122:321–338. doi: 10.1016/0022-2836(78)90193-6. [DOI] [PubMed] [Google Scholar]
- 41.Kalapos MP, Paulus H, Sarkar N. Identification of ribosomal protein S1 as a poly(A) binding protein in Escherichia coli. Biochimie. 1997;79:493–502. doi: 10.1016/S0300-9084(97)82741-1. [DOI] [PubMed] [Google Scholar]
- 42.Draper DE, von Hippel PH. Nucleic acid binding properties of Escherichia coli ribosomal protein S1. II. Co-operativity and specificity of binding site II. J Mol Biol. 1978;122:339–359. doi: 10.1016/0022-2836(78)90193-6. [DOI] [PubMed] [Google Scholar]
- 43.Schubert, O T. et al. Absolute Proteome Composition and Dynamics during Dormancy and Resuscitation of Mycobacterium tuberculosis. Cell Host Microbe18, 96–108 (2015). [DOI] [PubMed]
- 44.Ramadoss NS, et al. Small molecule inhibitors of trans-translation have broad-spectrum antibiotic activity. Proc Natl Acad Sci USA. 2013;110:10282–10287. doi: 10.1073/pnas.1302816110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Li, X., Hirano, R., Tagami, H. & Aiba, H. Protein tagging at rare codons is caused by tmRNA action at the 3′ end of nonstop mRNA generated in response to ribosome stalling. RNA12, 248–255 (2006). [DOI] [PMC free article] [PubMed]
- 46.Gimple, O. & Schön, A. In vitro and in vivo processing of cyanelle tmRNA by RNase P. Biol Chem 382, 1421–1429 (2001). [DOI] [PubMed]
- 47.Li Z, Pandit S, Deutscher MP. 3′ Exoribonucleolytic trimming is a common feature of the maturation of small, stable RNAs in Escherichia coli. Proc Natl Acad Sci USA. 1998;95:2856–2861. doi: 10.1073/pnas.95.6.2856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lin-Chao S, Wei C-L, Lin Y-T. RNase E is required for the maturation of ssrA RNA and normal ssrA RNA peptide-tagging activity. Proc Natl Acad Sci USA. 1999;96:12406–12411. doi: 10.1073/pnas.96.22.12406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Komine Y, Kitabatake M, Yokogawa T, Nishikawa K, Inokuchi H. A tRNA-like structure is present in 10Sa RNA, a small stable RNA from Escherichia coli. Proc Natl Acad Sci USA. 1994;91:9223–9227. doi: 10.1073/pnas.91.20.9223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Janssen BD, Hayes CS. Kinetics of Paused Ribosome Recycling in Escherichia coli. Journal of Molecular Biology. 2009;394:251–267. doi: 10.1016/j.jmb.2009.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yee, M., Gopal, P. & Dick, T. Missense Mutations in the Unfoldase ClpC1 of the Caseinolytic Protease Complex Are Associated with Pyrazinamide Resistance in Mycobacterium tuberculosis. Antimicrob Agents Chemother61, e02342–16 (2017). [DOI] [PMC free article] [PubMed]
- 52.Zhang S, et al. Mutation in clpC1 encoding an ATP-dependent ATPase involved in protein degradation is associated with pyrazinamide resistance in Mycobacterium tuberculosis. Emerg Microbes Infect. 2017;6:e8. doi: 10.1038/emi.2017.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


