Figure 1.
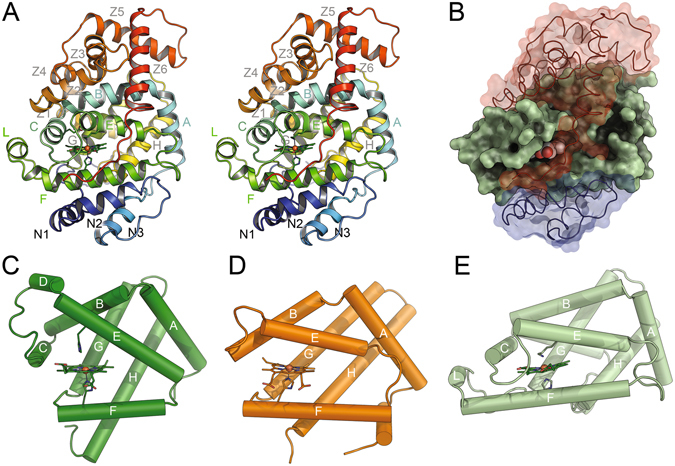
Lcp has a globin fold and similarities to globin-coupled sensor proteins. Three-dimensional structure of LcpK30 (stereo image), coloured from blue at the N-terminus to red at the C-terminus (A). Helix designations follow the standard globin nomenclature. Note that LcpK30 contains an additional helix, L, in the globin domain. Domain structure of LcpK30 (B). A central domain with a globin fold (green) is capped by an N-terminal domain with three helices (N1-3, blue) and a C-terminal domain consisting of 6 helices (Z1-6, red). Secondary structures of sperm whale myoglobin (PDB-ID 1MBN) (C), the globin-coupled sensor protein of G. sulfurreducens (PDB-ID 2W31) (D) and the central domain of LcpK30 (E) highlight the conserved topology of the globin folding core, as well as substantial variations in helix arrangement and haem accessibility. The haem groups including their axial ligands are shown as sticks in (A,C,D,E).
