Figure 2.
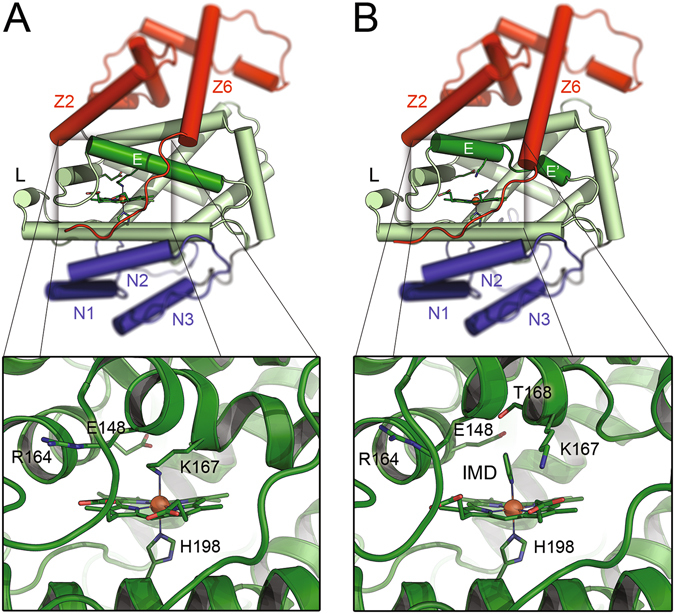
Two conformers of LcpK30. The LcpK30 structure exhibits an overall globin fold (A,B), and Lys167 serves as a distal axial ligand to the haem group, effectively preventing access for the substrate O2. The N-terminal extension with helices N1–N3 is shown in blue, the C-terminal with helices Z1–Z6 in red. In the presence of imidazole, an open form of LcpK30 was obtained (B). Lys167 is removed as a distal axial haem ligand and helix E is split into two fragments. Imidazole binds tightly to the haem and a continuous substrate channel passing the haem group is opened (Fig. 3).
