Figure 3.
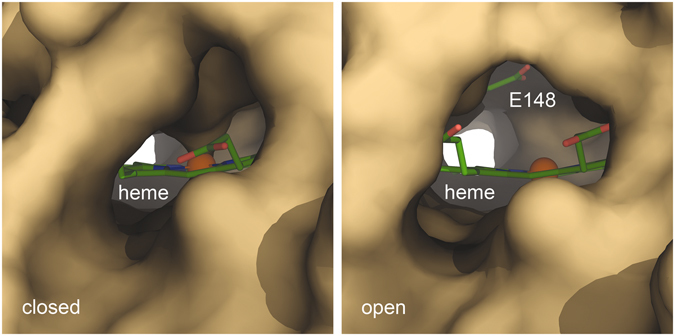
Comparison of the open and closed state of LcpK30. A molecular surface view of the hydrophobic channel passing the active site haem shows that the ligation of the iron ion by residue Lys167 effectively blocks the entrance. The open state provides a continuous channel that is lined by hydrophobic residues only, with the notable exception of Glu148 that possibly acts as a base during catalysis. The hydrophobicity of the channel seems to prevent access for water, leaving the haem in a five-coordinate state prior to O2 binding.
