Figure 6.
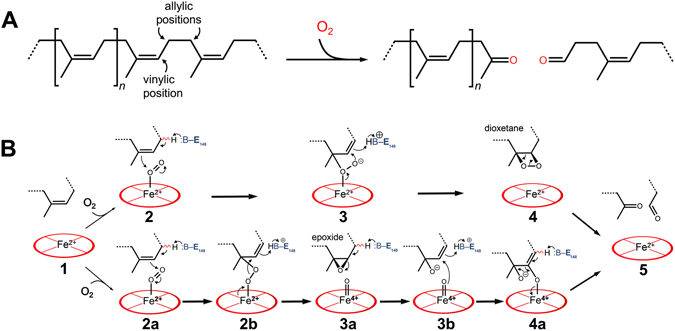
Mechanistic models of oxidative polyisoprene cleavage by LcpK30. Protons in allylic positions of poly (cis-1,4-isoprene) will be more acidic than those in vinylic positions (A). LcpAK30 catalyses the cleavage of the isoprenoid by inserting both oxygen atoms of an O2 molecule. Possible reaction mechanisms (B). Top: The substrate polymer is threaded into the channel of LcpK30 in the open state (1). O2 binds to the distal axial position of haem iron and a base in the channel, likely Glu148, abstracts a proton from an allylic position, leading to bond formation to an oxygen (2 + 2a). The second oxygen atom, with increased nucleophilic character, attacks the adjacent carbon (3), leading to the formation of an instable, cyclic dioxetane intermediate (4) that spontaneously rearranges to the cleaved product (5). Bottom: Alternatively, the haem-bound dioxygen can be cleaved (2b) to give a substrate epoxide and an oxo-ferryl intermediate (3a). The epoxide bond is cleaved by a nucleophilic attack of the oxo-ferryl-oxygen to the epoxide carbon atom (3b). Cleavage of the iron-oxygen bond (4a) leads to a release of the haem group and of the observed cleavage products (5).
