Abstract
Objective
Exposure to stressors alters activities of the adrenomedullary hormonal system (AHS), hypothalamic-pituitary-adrenocortical (HPA) axis, and sympathetic nervous system (SNS). Here we report results of a meta-analysis of the literature, examining inter-relationships among AHS, HPA, and SNS responses to stressors, as measured by plasma epinephrine (EPI), corticotrophin (ACTH), and norepinephrine (NE) levels.
Methods
The medical scientific literature was culled by PubMed searches, to retrieve publications describing original data about plasma EPI, ACTH, and NE levels measured before and during or after exposure to stressors. Magnitudes of responses were graded from a score of 0 for no response to 4 for a massive increase to ≥10 times the baseline value.
Results
A total of 15 stressors were identified for which at least 2 publications reported data for EPI, ACTH, and NE responses. A total of 60 reports were included. Mean EPI responses were strongly positively correlated with mean ACTH responses (r=0.93) and less strongly with NE responses (r=0.40). Plasma EPI responses were disproportionately larger than NE responses during hypoglycemia and smaller than NE responses during cold exposure without hypothermia, orthostasis, and active escape/avoidance. Plasma NE responses were disproportionately larger than ACTH responses during cold exposure without hypothermia and severe/exhausting exercise and smaller than ACTH responses during hypoglycemia.
Discussion
The results of this meta-analysis indicate a close association between adrenomedullary and hypothalamic-pituitary-adrenocortical responses across a variety of stressors. This association seems to be if anything stronger than that between adrenomedullary and sympathetic noradrenergic responses.
Keywords: Epinephrine, ACTH, Norepinephrine, Adrenomedullary, HPA, Sympathetic, Stress
It is by now well established that exposure to stressors alters activities of the adrenomedullary, adrenocortical, and sympathetic nervous systems. A sufficient number of studies have been published in which all three neuroendocrine systems have been monitored simultaneously to enable a meta-analysis of the literature, to examine inter-relationships among alterations in activities of these key effector systems upon exposure to different stressors and reassess long-standing concepts about these inter-relationships.
The first such concept is that there is a unitary “sympathoadrenal” system. In the early 20th century, the American physiologist, Walter B. Cannon, proposed that the sympathetic nervous system (SNS) and the adrenal gland hormone, “adrenin” (which came to be known as epinephrine [EPI] or adrenaline) function as a coordinated system maintaining homeostasis (a word he coined) during emergencies such as “fight or flight” situations (a phrase he introduced). According to Cannon’s concept, rapid activation of the sympathoadrenal system preserves the internal environment by producing compensatory and anticipatory adjustments that enhance the likelihood of survival. In 1939, Cannon formally proposed EPI as both the active principle of the adrenal gland and as the neurotransmitter of the sympathetic nervous system (Cannon and Lissak 1939), consistent with the functional unity of the sympathoadrenal system. The identity of the substance released at sympathetic nerve terminals remained controversial until 1946, when von Euler correctly identified norepinephrine (NE) as the sympathetic neurotransmitter in mammals (von Euler 1946).
A second, related concept is that there is a unitary stress response. Beginning in the 1930s, the East European physiologist, Hans Selye, popularized stress as a scientific idea (Selye 1936, 1956). Selye viewed all forms of stress as leading to (or being identical with) a stereotyped pathological pattern, including enlargement of the adrenal glands, shrinkage of the thymus gland (associated with atrophy of the lymph nodes and inhibition of inflammatory or immune responses), and ulcers or bleeding in the stomach or gastrointestinal tract. Selye defined stress as the nonspecific response of the body to any demand imposed upon it (Selye 1974). It was later demonstrated that these changes were associated with, and to at least some extent resulted from, activation of the hypothalamic-pituitary-adrenocortical (HPA) axis. Steroids released into the circulation from the adrenal cortex contribute to resistance but also be responsible for pathological changes. Selye’s concept that prolonged stress can produce physical disease and mental disorders is now widely accepted.
More than a half century elapsed before Selye’s doctrine of nonspecificity underwent experimental testing, which failed to confirm it (Pacak et al. 1998). Nevertheless, modern lay literature and medical websites continue to accept the notion of a unitary stress response. For instance, a Google search yielded about 14,900,000 hits for “the stress response.” According to Yahoo/Health, “The stress response is the set of physical and emotional changes the human body makes in response to a threat or stress. It sometimes is called the “fight-or-flight” response.” (As indicated above, it was Cannon who introduced this phrase.)
After adequately sensitive assay methods for plasma levels of NE and EPI became available, evidence rapidly accumulated for different noradrenergic vs. adrenergic responses in different situations (Cryer 1980; Robertson et al. 1979; Young et al. 1984). A new concept began to emerge, in which the SNS plays key roles in appropriate redistribution of blood flows in situations such as orthostasis, cold exposure, mild blood loss, locomotion, exercise, altered salt intake, and water immersion; and the adrenomedullary hormonal system (AHS) responds to global or metabolic threats, such as hypoglycemia, hemorrhagic hypotension, exercise beyond an anaerobic threshold, asphyxiation, emotional distress, and shock. Evidence also accumulated for an association of SNS activation with active escape, avoidance, or attack and an association of AHS activation with passive, immobile fear.
More generally, according to a recently proposed concept, stress responses have a kind of “primitive specificity,” and the AHS and SNS can respond differentially, depending on the type and intensity of the stressor as sensed by the organism and interpreted in light of experience (Goldstein 1995). Instead of the SNS becoming active only in emergencies, tonic sympathetic nervous outflow to several vascular beds, organs, and glands is present even under resting conditions, and everyday experiences such as orthostasis, locomotion, the post-prandial state, and exposure to altered environmental temperature can alter sympathoneural outflows, with little or no activation of the AHS. On the other hand, even a slight amount of glucoprivation, posing an overall metabolic challenge, evokes mainly an adrenomedullary response, without generalized SNS activation.
A third concept considered in this meta-analyses is that in response to situations that stimulate adrenomedullary secretion, there is concurrent activation of the HPA axis. The association of AHS with HPA activation might be even closer than that of AHS with SNS activation. Testing this concept requires analysis of experiments in which AHS, HPA, and SNS responses to various stressors are assessed simultaneously. This formal meta-analysis comprehensively assessed the clinical and preclinical literature in which plasma EPI, ACTH, and NE responses to stressors were measured in the same studies.
Methods
The medical scientific literature was culled by multiple computer searches of PubMed. The searches were designed to retrieve publications describing original data about plasma EPI, ACTH, and NE levels measured before and during or after exposure to stressors. The searches were followed by retrieving the reviewing printed research reports.
Magnitudes of responses were categorized according to the following criteria. If there was no significant change in the plasma levels of the dependent variable, a score of 0 was assigned. If there was a statistically significant increase, but less than a doubling, of the pre-stress baseline level, a score of 1 was assigned. If there was at least a doubling of the baseline value, up 3 times the baseline value, a score or 2 was assigned. If there were a large increase, from 3 up to 10 times the baseline value, a score of 3 was assigned. If there was a massive increase to ≥10 times the baseline value, a score of 4 was assigned.
For each stressor, the average across studies was used, without weighting studies by numbers of subjects.
As indicated in Table 1, a total of 15 different stressors were identified for which the available literature satisfied the above criteria.
Table 1. Stressors in this review.
Stressors are listed in approximate descending order of adrenomedullary activation (numbers in italics are numbers of studies for each stressor)
|
Results
A total of 15 stressor categories and a total of 60 studies were included in the meta-analysis (Tables 1 and 2). The most frequently studied stressors were hypoglycemia and hemorrhagic hypotension. Although there was literature about other categories of stressor, such as cardiac arrest, pain, public performance, blood loss without hypotension, and mild exercise, the available literature did not meet the inclusion criteria for the meta-analysis.
Table 2.
Magnitudes of Responses to Stressors, from No Response (0) to Extremely Large Response (4) Values in boldface are means for each category.
| Stressor | EPI | ACTH | NE | Reference | Notes |
|---|---|---|---|---|---|
| Hypoglycemia | 4 | 2 | 1 | Costa et al. 1993 | Humans |
| Hypoglycemia | 4 | 3 | 0 | Giordano et al. 2003 | Humans |
| Hypoglycemia | 4 | 2 | 1 | Mcgregor et al. 2002 | Humans. [Cortisol] |
| Hypoglycemia | 4 | 4 | 2 | Pacak et al. 1998 | Rats |
| Hypoglycemia | 4 | 2 | 0 | Radikova et al. 2003 | Humans |
| Hypoglycemia | 4 | 2 | 2 | Schmid et al. 2007 | Humans |
| Hypoglycemia | 3 | 3 | 1 | Toso et al. 1993 | Dogs |
| Hypoglycemia | 4 | 3 | 1 | Watabe et al. 1987 | Humans |
| Hypoglycemia | 3.9 | 2.6 | 1.0 | ||
| Immobilization | 4 | 4 | 3 | Dronjak et al. 2004 | Rats |
| Immobilization | 4 | 3 | 3 | Jezova et al. 1999 | Rats |
| Immobilization | 4 | 4 | 3 | Pacak et al. 1998 | Rats |
| Immobilization | 3 | 2 | 2 | Tjurmina et al. 2002 | Rats |
| Immobilization | 3.8 | 3.3 | 2.8 | ||
| Hemorrh. Hypotens. | 4 | 3 | 3 | Bereiter et al. 1986 | Cats |
| Hemorrh. Hypotens. | 4 | 3 | 2 | Darlington et al. 1986 | Rats |
| Hemorrh. Hypotens. | 4 | 4 | 2 | Grassler et al. 1990 | Rats |
| Hemorrh. Hypotens. | 4 | 3 | 3 | Molina 2001 | Rats |
| Hemorrh. Hypotens. | 1 | 4 | 1 | Pacak et al. 1998 | Rats |
| Hemorrh. Hypotens. | 4 | 3 | 4 | Wade et al. 1991 | Swine |
| Hemorrh. Hypotens. | 3.5 | 3.3 | 2.5 | ||
| Exercise, Severe | 4 | 4 | 4 | Deuster et al. 1989 | Humans |
| Exercise, Severe | 4 | 4 | 4 | Nagata et al. 1999 | Horses |
| Exercise, Severe | 3 | 3 | 3 | Oleshansky et al. 1990 | Humans |
| Exercise, Severe | 2 | 3 | 3 | Schwarz & Kindermann 1990 | Humans |
| Exercise, Severe | 3.3 | 3.5 | 3.5 | ||
| Electric shock | 3 | 3 | 3 | de Boer et al. 1990 | Rats. [Corticosterone] |
| Electric shock | 3 | 4 | 3 | Thiagarajan et al. 1989 | Rats |
| Electric shock | 4 | 3 | 3 | Weinger et al. 1991 | Humans |
| Electric shock | 3.3 | 3.3 | 3.0 | ||
| Social Stress | 4 | 3 | 3 | Ayala et al. 2004 | Monkeys |
| Social Stress | 2 | 1 | 2 | Habib et al. 2000 | Monkeys |
| Social Stress | 3.0 | 2.0 | 2.5 | ||
| Fainting | 2 | 2 | 0 | Carroll et al. 1995 | Humans |
| Fainting | 3 | 3 | 3 | Gasiorowska et al. 2005 | Humans |
| Fainting | 3 | 3 | 2 | Jardine et al. 1997 | Humans |
| Fainting | 2.7 | 2.7 | 1.7 | ||
| Handling | 2 | 2 | 2 | Dobrakovova et al. 1993 | Rats |
| Handling | 2 | 1 | 1 | Makatsori et al. 2005 | Rats |
| Handling | 2.0 | 1.5 | 1.5 | ||
| Fear | 3 | 2 | 3 | Korte et al. 1992 | Rats |
| Fear | 3 | 3 | 3 | Nijsen et al. 2000 | Rats |
| Fear | 0 | 1 | 0 | Pitman et al. 1992 | Rats |
| Fear | 1 | 0 | 0 | Pitman et al. 1995 | Rats |
| Fear | 1.8 | 1.5 | 1.5 | ||
| Surgery | 0 | 0 | 0 | Chi et al. 2001 | Humans. Neurosurg. |
| Surgery | 3 | 3 | 2 | Donald et al. 1993 | Humans |
| Surgery | 1 | 1 | 2 | Kudoh et al. 1999 | Humans |
| Surgery | 2 | 2 | 1 | Nguyen et al. 2002 | Humans |
| Surgery | 1 | 1 | 2 | Udelsman et al. 1987 | Humans |
| Surgery | 1.4 | 1.4 | 1.4 | ||
| Mild Hypothermia | 2 | 2 | 2 | Frank et al. 1995 | Humans. Post-op. |
| Mild Hypothermia | 0 | 2 | 0 | Chi et al. 2001 | Humans. Neurosurg. |
| Mild Hypothermia | 1.0 | 2.0 | 1.0 | ||
| Lab. Mental Challenge | 0 | 0 | 0 | Costa et al. 1993 | Humans |
| Lab. Mental Challenge | 0 | 0 | 0 | Gerra et al. 2000 | Humans |
| Lab. Mental Challenge | 2 | 1 | 2 | Gerra et al. 2001 | Humans |
| Lab. Mental Challenge | 1 | 2 | 1 | Schommer et al. 2003 | Humans |
| Lab. Mental Challenge | 2 | 1 | 1 | Yoshiuchi et al. 1997 | Humans |
| Lab. Mental Challenge | 1.0 | 0.8 | 0.8 | ||
| Active Escape/Avoidance | 1 | 2 | 2 | de Boer et al. 1990 | Rats |
| Active Escape/Avoidance | 1 | 0 | 2 | Korte et al. 1992 | Rats |
| Active Escape/Avoidance | 1.0 | 1.0 | 2.0 | ||
| Orthostasis | 0 | 0 | 2 | Carroll et al. 1995 | Humans |
| Orthostasis | 1 | 0 | 1 | Gasiorowska et al. 2005 | Humans. LBNP |
| Orthostasis | 0 | 0 | 1 | Jardine et al. 1997 | Humans |
| Orthostasis | 0 | 0 | 1 | Mlynarik et al. 2007 | Humans |
| Orthostasis | 1 | 2 | 2 | Radikova et al. 2003 | Humans |
| Orthostasis | 0.4 | 0.4 | 1.4 | ||
| Cold, No Hypotherm. | 0 | 0 | 3 | Fukuhara et al. 1996 | Rats |
| Cold, No Hypotherm. | 0 | 0 | 3 | Leppaluoto et al. 2008 | Humans |
| Cold, No Hypotherm. | 0 | 0 | 2 | Marino et al. 1998 | Humans. [Cortisol] |
| Cold, No Hypotherm. | 0 | 0 | 3 | Pacak et al. 1998 | Rats |
| Cold, No Hypotherm. | 0 | 0 | 2 | Wittert et al. 1992 | Humans |
| Cold, No Hypotherm. | 0.0 | 0.0 | 2.6 |
Abbreviations: AHS=adrenomedullary hormonal system; HPA=hypothalamic-pituitary-adrenocortical system; SNS=sympathetic noradrenergic system; LBNP=lower body negative pressure; ACTH=corticotrophin
Across the 15 stressors, mean plasma EPI responses (Table 2) ranged from 0.0 (cold exposure, no hypothermia) to 3.9 (hypoglycemia), ACTH responses ranged from 0.0 (cold exposure, no hypothermia) to 3.5 (exercise, severe/exhaustion), and NE responses ranged from 1.0 (hypoglycemia) to 3.5 (exercise, severe/exhaustion). Therefore, there was a substantial range of response intensities for all three dependent variables.
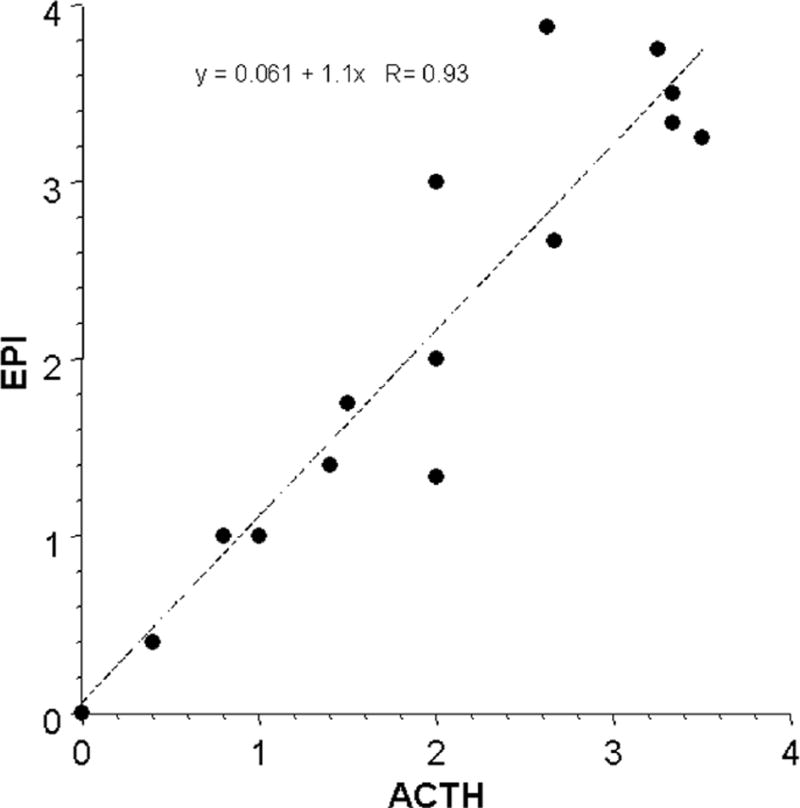
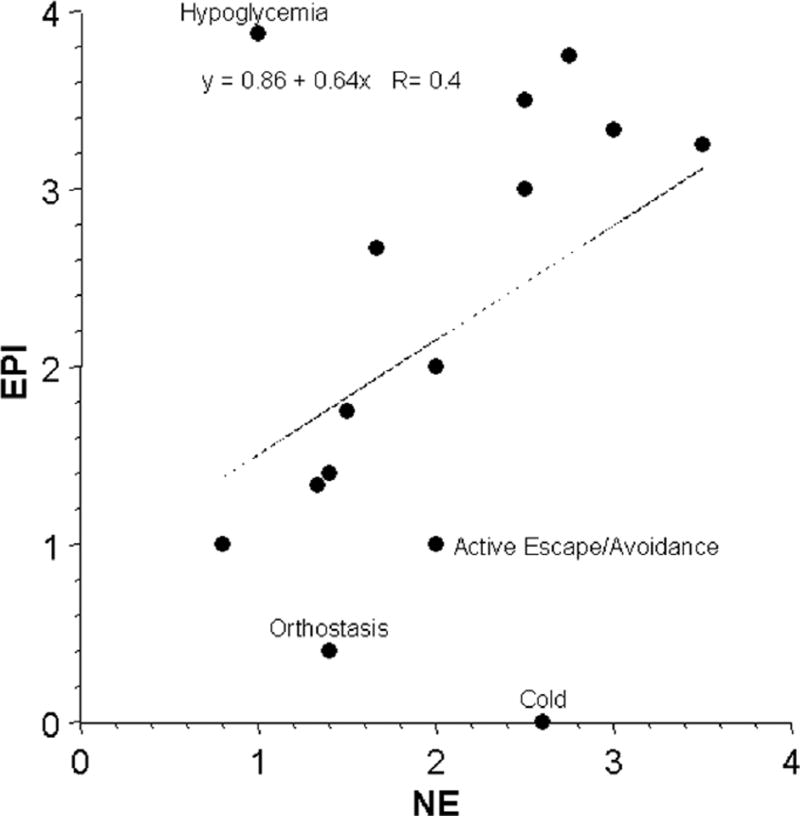
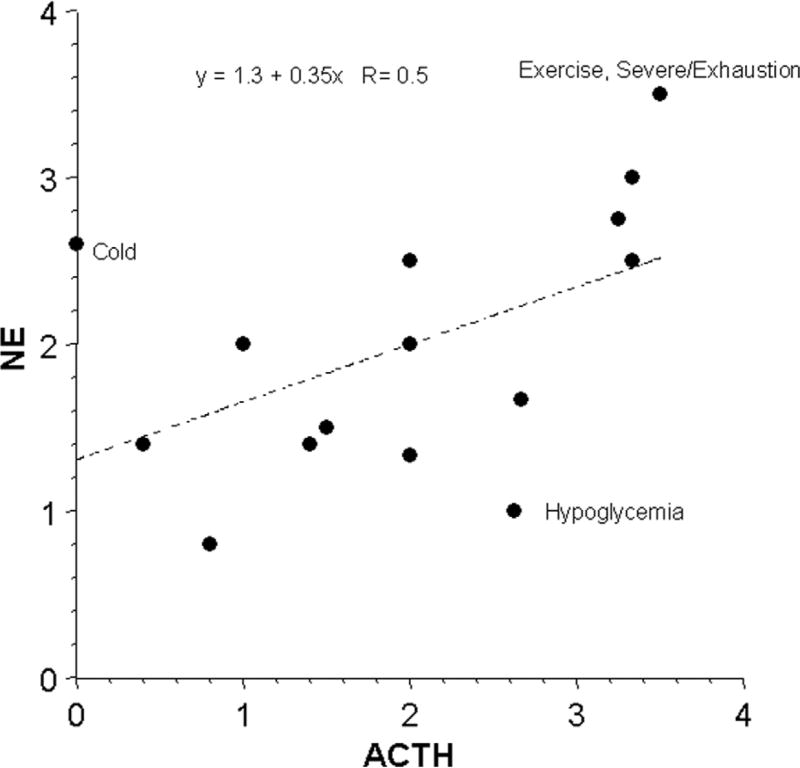
Mean EPI responses were strongly positively correlated with mean ACTH responses (Fig. 1) and less strongly with NE responses (Fig. 2). Plasma EPI responses were disproportionately larger than NE responses during hypoglycemia and smaller than NE responses during cold exposure without hypothermia, orthostasis, and active escape/avoidance. Plasma NE responses were disproportionately larger than expected for ACTH responses during cold exposure without hypothermia and severe/exhausting exercise and smaller than ACTH responses during hypoglycemia (Fig. 3).
Fig 1.
Mean values for plasma levels of epinephrine (EPI) and corticotrophin (ACTH) across 15 different stressors. Equation is for the line of best fit.
Fig 2.
Mean values for responses of plasma levels of epinephrine (EPI) and norepinephrine (NE) across 15 different stressors. Equation is for the line of best fit.
Fig 3.
Mean values for responses of plasma levels of norepinephrine (NE) and corticotrophin (ACTH) across 15 different stressors. Equation is for the line of best fit.
Discussion
The results of this meta-analysis indicate a close association between adrenomedullary and hypothalamic-pituitary-adrenocortical responses across a variety of stressors. This association seems to be if anything stronger than that between adrenomedullary and sympathetic noradrenergic responses. The findings therefore favor the concept of a unitary adrenal system over that of a unitary sympathoadrenal system.
The analysis also supports the notion of “primitive specificity,” according to which stress responses occur in relatively specific neuroendocrine patterns. By promoting homeostasis, such patterning would have provided clear advantages in natural selection and therefore evolved. In contrast, Cannon’s and Selye’s theories, based as they are on the same stereotyped responses regardless of the stress, do not account adequately for outliers in the scatter plots relating EPI to NE and NE to ACTH responses. For instance, plasma EPI and ACTH responses to hypoglycemia are disproportionately larger than NE responses, and plasma NE responses to cold exposure without hypothermia are disproportionately larger than EPI or ACTH responses.
The largest AHS responses were reported for stressors that can be categorized in terms of posing a global or metabolic threat. There are several other stressors that should also pose this type of challenge to organismic integrity—pain, endotoxemia, and cardiogenic shock are examples; however, the studies culled on these topics did not fit the criteria for inclusion in the meta-analysis. Subcutaneous injection of formalin evokes relatively large adrenomedullary compared to sympathoneural responses in rats (Pacak et al. 1998). Administration of interleukin-1 elicits about a 5-fold increase in ACTH, 3-fold increase in EPI, and 2-fold increase in plasma NE (Berkenbosch et al. 1989). Cardiac arrest results in massive increases in EPI levels and substantial increases in cortisol and NE levels (Foley et al. 1987), patients undergoing cardiopulmonary resuscitation have high EPI, ACTH, and NE levels (Lindner et al. 1996), and stress cardiopathy is associated with extraordinarily high plasma EPI and NE levels (Wittstein et al. 2005).
Meta-analysis of the available literature therefore supports a closer association of adrenomedullary with HPA than with SNS responses across a variety of stressors. There seems to be at least as good justification for the concept of coordinated adrenocortical-adrenomedullary responses as for coordinated adrenomedullary-sympathoneural responses.
Acknowledgments
This research was supported by the intramural research program of the NINDS, NIH.
References
- Ayala AR, Pushkas J, Higley JD, Ronsaville D, Gold PW, Chrousos GP, Pacak K, Calis KA, Gerald M, Lindell S, Rice KC, Cizza G. Behavioral, adrenal, and sympathetic responses to long-term administration of an oral corticotropin-releasing hormone receptor antagonist in a primate stress paradigm. J Clin Endocrinol Metab. 2004;89:5729–5737. doi: 10.1210/jc.2003-032170. [DOI] [PubMed] [Google Scholar]
- Bereiter DA, Zaid AM, Gann DS. Effect of rate of hemorrhage on sympathoadrenal catecholamine release in cats. Am J Physiol. 1986;250:E69–E75. doi: 10.1152/ajpendo.1986.250.1.E69. [DOI] [PubMed] [Google Scholar]
- Berkenbosch F, De Goeij DE, Rey AD, Besedovsky HO. Neuroendocrine, sympathetic and metabolic responses induced by interleukin-1. Neuroendocrinology. 1989;50:570–576. doi: 10.1159/000125283. [DOI] [PubMed] [Google Scholar]
- Cannon WB, Lissak K. Evidence for adrenaline in adrenergic neurones. Am. J. Physiol. 1939;125:765–777. [Google Scholar]
- Carroll JF, Wood CE, Pollock ML, Graves JE, Convertino VA, Lowenthal DT. Hormonal responses in elders experiencing pre-syncopal symptoms during head-up tilt before and after exercise training. J Gerontol A Biol Sci Med Sci. 1995;50:M324–M329. doi: 10.1093/gerona/50a.6.m324. [DOI] [PubMed] [Google Scholar]
- Chi OZ, Choi YK, Lee DI, Kim YS, Lee I. Intraoperative mild hypothermia does not increase the plasma concentration of stress hormones during neurosurgery. Can. J. Anaesth. 2001;48:815–818. doi: 10.1007/BF03016700. [DOI] [PubMed] [Google Scholar]
- Costa A, Martignoni E, Blandini F, Petraglia F, Genazzani AR, Nappi G. Effects of etoperidone on sympathetic and pituitary-adrenal responses to diverse stressors in humans. Clin Neuropharmacol. 1993;16:127–138. doi: 10.1097/00002826-199304000-00005. [DOI] [PubMed] [Google Scholar]
- Cryer PE. Physiology and pathophysiology of the human sympathoadrenal neuroendocrine system. N. Engl. J. Med. 1980;303:436–444. doi: 10.1056/NEJM198008213030806. [DOI] [PubMed] [Google Scholar]
- Darlington DN, Shinsako J, Dallman MF. Responses of ACTH, epinephrine, norepinephrine, and cardiovascular system to hemorrhage. Am J Physiol. 1986;251:H612–H618. doi: 10.1152/ajpheart.1986.251.3.H612. [DOI] [PubMed] [Google Scholar]
- de Boer SF, Slangen JL, Van Der Gugten J. Plasma catecholamine and corticosterone levels during active and passive shock-prod avoidance behavior in rats: effects of chlordiazepoxide. Physiol. Behav. 1990;47:1089–1098. doi: 10.1016/0031-9384(90)90357-a. [DOI] [PubMed] [Google Scholar]
- Deuster PA, Chrousos GP, Luger A, DeBolt JE, Bernier LL, Trostmann UH, Kyle SB, Montgomery LC, Loriaux DL. Hormonal and metabolic responses of untrained, moderately trained, and highly trained men to three exercise intensities. Metabolism. 1989;38:141–148. doi: 10.1016/0026-0495(89)90253-9. [DOI] [PubMed] [Google Scholar]
- Dobrakovova M, Kvetnansky R, Oprsalova Z, Jezova D. Specificity of the effect of repeated handling on sympathetic-adrenomedullary and pituitary-adrenocortical activity in rats. Psychoneuroendocrinology. 1993;18:163–174. doi: 10.1016/0306-4530(93)90001-2. [DOI] [PubMed] [Google Scholar]
- Donald RA, Perry EG, Wittert GA, Chapman M, Livesey JH, Ellis MJ, Evans MJ, Yandle T, Espiner EA. The plasma ACTH, AVP, CRH and catecholamine responses to conventional and laparoscopic cholecystectomy. Clin Endocrinol (Oxf) 1993;38:609–615. doi: 10.1111/j.1365-2265.1993.tb02142.x. [DOI] [PubMed] [Google Scholar]
- Dronjak S, Gavrilovic L, Filipovic D, Radojcic MB. Immobilization and cold stress affect sympatho-adrenomedullary system and pituitary-adrenocortical axis of rats exposed to long-term isolation and crowding. Physiol Behav. 2004;81:409–415. doi: 10.1016/j.physbeh.2004.01.011. [DOI] [PubMed] [Google Scholar]
- Foley PJ, Tacker WA, Wortsman J, Frank S, Cryer PE. Plasma catecholamine and serum cortisol responses to experimental cardiac arrest in dogs. Am. J. Physiol. 1987;253:E283–E289. doi: 10.1152/ajpendo.1987.253.3.E283. [DOI] [PubMed] [Google Scholar]
- Frank SM, Higgins MS, Breslow MJ, Fleisher LA, Gorman RB, Sitzmann JV, Raff H, Beattie C. The catecholamine, cortisol, and hemodynamic responses to mild perioperative hypothermia. A randomized clinical trial. Anesthesiology. 1995;82:83–93. doi: 10.1097/00000542-199501000-00012. [DOI] [PubMed] [Google Scholar]
- Fukuhara K, Kvetnansky R, Cizza G, Pacak K, Ohara H, Goldstein DS, Kopin IJ. Interrelations between sympathoadrenal system and hypothalamo-pituitary-adrenocortical/thyroid systems in rats exposed to cold stress. J Neuroendocrinol. 1996;8:533–541. doi: 10.1046/j.1365-2826.1996.04877.x. [DOI] [PubMed] [Google Scholar]
- Gasiorowska A, Nazar K, Mikulski T, Cybulski G, Niewiadomski W, Smorawinski J, Krzeminski K, Porta S, Kaciuba-Uscilko H. Hemodynamic and neuroendocrine predictors of lower body negative pressure (LBNP) intolerance in healthy young men. J Physiol Pharmacol. 2005;56:179–193. [PubMed] [Google Scholar]
- Gerra G, Zaimovic A, Mascetti GG, Gardini S, Zambelli U, Timpano M, Raggi MA, Brambilla F. Neuroendocrine responses to experimentally-induced psychological stress in healthy humans. Psychoneuroendocrinology. 2001;26:91–107. doi: 10.1016/s0306-4530(00)00046-9. [DOI] [PubMed] [Google Scholar]
- Gerra G, Zaimovic A, Zambelli U, Timpano M, Reali N, Bernasconi S, Brambilla F. Neuroendocrine responses to psychological stress in adolescents with anxiety disorder. Neuropsychobiology. 2000;42:82–92. doi: 10.1159/000026677. [DOI] [PubMed] [Google Scholar]
- Giordano R, Grottoli S, Brossa P, Pellegrino M, Destefanis S, Lanfranco F, Gianotti L, Ghigo E, Arvat E. Alprazolam (a benzodiazepine activating GABA receptor) reduces the neuroendocrine responses to insulin-induced hypoglycaemia in humans. Clin Endocrinol (Oxf) 2003;59:314–320. doi: 10.1046/j.1365-2265.2003.01847.x. [DOI] [PubMed] [Google Scholar]
- Goldstein DS. Stress, Catecholamines, and Cardiovascular Disease. Oxford University Press; New York: 1995. [Google Scholar]
- Grassler J, Jezova D, Kvetnansky R, Scheuch DW. Hormonal responses to hemorrhage and their relationship to individual hemorrhagic shock susceptibility. Endocrinol Exp. 1990;24:105–116. [PubMed] [Google Scholar]
- Habib KE, Weld KP, Rice KC, Pushkas J, Champoux M, Listwak S, Webster EL, Atkinson AJ, Schulkin J, Contoreggi C, Chrousos GP, Mccann SM, Suomi SJ, Higley JD, Gold PW. Oral administration of a corticotropin-releasing hormone receptor antagonist significantly attenuates behavioral, neuroendocrine, and autonomic responses to stress in primates. Proc Natl Acad Sci U S A. 2000;97:6079–6084. doi: 10.1073/pnas.97.11.6079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jardine DL, Melton IC, Crozier IG, Bennett SI, Donald RA, Ikram H. Neurohormonal response to head-up tilt and its role in vasovagal syncope. Am J Cardiol. 1997;79:1302–1306. doi: 10.1016/s0002-9149(9x)00084-9. [DOI] [PubMed] [Google Scholar]
- Jezova D, Ochedalski T, Glickman M, Kiss A, Aguilera G. Central corticotropin-releasing hormone receptors modulate hypothalamic-pituitary-adrenocortical and sympathoadrenal activity during stress. Neuroscience. 1999;94:797–802. doi: 10.1016/s0306-4522(99)00333-4. [DOI] [PubMed] [Google Scholar]
- Korte SM, Bouws GA, Koolhaas JM, Bohus B. Neuroendocrine and behavioral responses during conditioned active and passive behavior in the defensive burying/probe avoidance paradigm: effects of ipsapirone. Physiol Behav. 1992;52:355–361. doi: 10.1016/0031-9384(92)90284-9. [DOI] [PubMed] [Google Scholar]
- Kudoh A, Ishihara H, Matsuki A. Response to surgical stress in elderly patients and Alzheimer’s disease. Can J Anaesth. 1999;46:247–252. doi: 10.1007/BF03012604. [DOI] [PubMed] [Google Scholar]
- Leppaluoto J, Westerlund T, Huttunen P, Oksa J, Smolander J, Dugue B, Mikkelsson M. Effects of long-term whole-body cold exposures on plasma concentrations of ACTH, beta-endorphin, cortisol, catecholamines and cytokines in healthy females. Scand J Clin Lab Invest. 2008;68:145–153. doi: 10.1080/00365510701516350. [DOI] [PubMed] [Google Scholar]
- Lindner KH, Haak T, Keller A, Bothner U, Lurie KG. Release of endogenous vasopressors during and after cardiopulmonary resuscitation. Heart. 1996;75:145–150. doi: 10.1136/hrt.75.2.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makatsori A, Michal D, Eduard U, Bakos J, Jezova D. Neuroendocrine changes in adult female rats prenatally exposed to phenytoin. Neurotoxicol Teratol. 2005;27:509–514. doi: 10.1016/j.ntt.2005.01.012. [DOI] [PubMed] [Google Scholar]
- Marino F, Sockler JM, Fry JM. Thermoregulatory, metabolic and sympathoadrenal responses to repeated brief exposure to cold. Scand J Clin Lab Invest. 1998;58:537–545. doi: 10.1080/00365519850186157. [DOI] [PubMed] [Google Scholar]
- McGregor VP, Banarer S, Cryer PE. Elevated endogenous cortisol reduces autonomic neuroendocrine and symptom responses to subsequent hypoglycemia. Am J Physiol Endocrinol Metab. 2002;282:E770–E777. doi: 10.1152/ajpendo.00447.2001. [DOI] [PubMed] [Google Scholar]
- Mlynarik M, Makatsori A, Dicko I, Hinghofer-Szalkay HG, Jezova D. Postural changes associated with public speech tests lead to mild and selective activation of stress hormone release. J Physiol Pharmacol. 2007;58:95–103. [PubMed] [Google Scholar]
- Molina PE. Opiate modulation of hemodynamic, hormonal, and cytokine responses to hemorrhage. Shock. 2001;15:471–478. doi: 10.1097/00024382-200115060-00011. [DOI] [PubMed] [Google Scholar]
- Nagata S, Takeda F, Kurosawa M, Mima K, Hiraga A, Kai M, Taya K. Plasma adrenocorticotropin, cortisol and catecholamines response to various exercises. Equine Vet J Suppl. 1999;30:570–574. doi: 10.1111/j.2042-3306.1999.tb05286.x. [DOI] [PubMed] [Google Scholar]
- Nguyen NT, Goldman CD, Ho HS, Gosselin RC, Singh A, Wolfe BM. Systemic stress response after laparoscopic and open gastric bypass. J Am Coll Surg. 2002;194:557–566. doi: 10.1016/s1072-7515(02)01132-8. [DOI] [PubMed] [Google Scholar]
- Nijsen MJ, Croiset G, Diamant M, Stam R, Kamphuis PJ, Bruijnzeel A, de Wied D, Wiegant VM. Endogenous corticotropin-releasing hormone inhibits conditioned-fear-induced vagal activation in the rat. Eur J Pharmacol. 2000;389:89–98. doi: 10.1016/s0014-2999(99)00870-5. [DOI] [PubMed] [Google Scholar]
- Oleshansky MA, Zoltick JM, Herman RH, Mougey EH, Meyerhoff JL. The influence of fitness on neuroendocrine responses to exhaustive treadmill exercise. Eur J Appl Physiol Occup Physiol. 1990;59:405–410. doi: 10.1007/BF02388620. [DOI] [PubMed] [Google Scholar]
- Pacak K, Palkovits M, Yadid G, Kvetnansky R, Kopin IJ, Goldstein DS. Heterogeneous neurochemical responses to different stressors: A test of Selye’s doctrine of nonspecificity. Am. J. Physiol. 1998;275:R1247–R1255. doi: 10.1152/ajpregu.1998.275.4.R1247. [DOI] [PubMed] [Google Scholar]
- Pitman DL, Natelson BH, Ottenweller JE. Classical aversive conditioning of catecholamine and corticosterone responses. Integr Physiol Behav Sci. 1992;27:13–22. doi: 10.1007/BF02691088. [DOI] [PubMed] [Google Scholar]
- Pitman DL, Natelson BH, Ottenweller JE, McCarty R, Pritzel T, Tapp WN. Effects of exposure to stressors of varying predictability on adrenal function in rats. Behav Neurosci. 1995;109:767–776. doi: 10.1037//0735-7044.109.4.767. [DOI] [PubMed] [Google Scholar]
- Radikova Z, Penesova A, Jezova D, Kvetnansky R, Vigas M, Macho L, Koska J. Body position and the neuroendocrine response to insulin-induced hypoglycemia in healthy subjects. Arch Physiol Biochem. 2003;111:399–405. doi: 10.3109/13813450312331337658. [DOI] [PubMed] [Google Scholar]
- Robertson DA, Johnson GA, Robertson RM, Nies AS, Shand DG, Oates JA. Comparative assessment of stimuli that release neuronal and adrenomedullary catecholamines in man. Circulation. 1979;59:637–643. doi: 10.1161/01.cir.59.4.637. [DOI] [PubMed] [Google Scholar]
- Schmid SM, Hallschmid M, Jauch-Chara K, Bandorf N, Born J, Schultes B. Sleep loss alters basal metabolic hormone secretion and modulates the dynamic counterregulatory response to hypoglycemia. J Clin Endocrinol Metab. 2007;92:3044–3051. doi: 10.1210/jc.2006-2788. [DOI] [PubMed] [Google Scholar]
- Schommer NC, Hellhammer DH, Kirschbaum C. Dissociation between reactivity of the hypothalamus-pituitary-adrenal axis and the sympathetic-adrenal-medullary system to repeated psychosocial stress. Psychosom Med. 2003;65:450–460. doi: 10.1097/01.psy.0000035721.12441.17. [DOI] [PubMed] [Google Scholar]
- Schwarz L, Kindermann W. Beta-endorphin, adrenocorticotropic hormone, cortisol and catecholamines during aerobic and anaerobic exercise. Eur J Appl Physiol Occup Physiol. 1990;61:165–171. doi: 10.1007/BF00357593. [DOI] [PubMed] [Google Scholar]
- Selye H. A syndrome produced by diverse nocuous agents. Nature. 1936;138:32. doi: 10.1176/jnp.10.2.230a. [DOI] [PubMed] [Google Scholar]
- Selye H. Book Stress without Distress. New American Library; New York: 1974. [Google Scholar]
- Selye H. Book The Stress of Life. McGraw-Hill; New York: 1956. [Google Scholar]
- Thiagarajan AB, Gleiter CH, Mefford IN, Eskay RL, Nutt DJ. Effect of single and repeated electroconvulsive shock on the hypothalamic-pituitary-adrenal axis and plasma catecholamines in rats. Psychopharmacology (Berl) 1989;97:548–552. doi: 10.1007/BF00439562. [DOI] [PubMed] [Google Scholar]
- Tjurmina OA, Armando I, Saavedra JM, Goldstein DS, Murphy DL. Exaggerated adrenomedullary response to immobilization in mice with targeted disruption of the serotonin transporter gene. Endocrinology. 2002;143:4520–4526. doi: 10.1210/en.2002-220416. [DOI] [PubMed] [Google Scholar]
- Toso CF, Rodriguez RR, Renauld AR, Marquez AG, Linares LM. Adrenocorticotrophic hormone, cortisol and catecholamine concentrations during insulin hypoglycaemia in dogs anaesthetized with thiopentone. Can J Anaesth. 1993;40:1084–1091. doi: 10.1007/BF03009482. [DOI] [PubMed] [Google Scholar]
- Udelsman R, Norton JA, Jelenich SE, Goldstein DS, Linehan WM, Loriaux DL, Chrousos GP. Responses of the hypothalamic-pituitary-adrenal and renin- angiotensin axes and the sympathetic system during controlled surgical and anesthetic stress. J. Clin. Endocrinol. Metab. 1987;64:986–994. doi: 10.1210/jcem-64-5-986. [DOI] [PubMed] [Google Scholar]
- Von Euler US. A specific sympathomimetic ergone in adrenergic nerve fibres (sympathin) and its relations to adrenaline and nor-adrenaline. Acta Physiol Scand. 1946;12:73–96. [Google Scholar]
- Wade CE, Hannon JP, Bossone CA, Hunt MM, Loveday JA, Coppes RI, Jr, Gildengorin VL. Neuroendocrine responses to hypertonic saline/dextran resuscitation following hemorrhage. Circ Shock. 1991;35:37–43. [PubMed] [Google Scholar]
- Watabe T, Tanaka K, Kumagae M, Itoh S, Takeda F, Morio K, Hasegawa M, Horiuchi T, Miyabe S, Shimizu N. Hormonal responses to insulin-induced hypoglycemia in man. J Clin Endocrinol Metab. 1987;65:1187–1191. doi: 10.1210/jcem-65-6-1187. [DOI] [PubMed] [Google Scholar]
- Weinger MB, Partridge BL, Hauger R, Mirow A, Brown M. Prevention of the cardiovascular and neuroendocrine response to electroconvulsive therapy: II. Effects of pretreatment regimens on catecholamines, ACTH, vasopressin, and cortisol. Anesth Analg. 1991;73:563–569. [PubMed] [Google Scholar]
- Wittert GA, Or HK, Livesey JH, Richards AM, Donald RA, Espiner EA. Vasopressin, corticotrophin-releasing factor, and pituitary adrenal responses to acute cold stress in normal humans. J Clin Endocrinol Metab. 1992;75:750–755. doi: 10.1210/jcem.75.3.1517364. [DOI] [PubMed] [Google Scholar]
- Wittstein IS, Thiemann DR, Lima JA, Baughman KL, Schulman SP, Gerstenblith G, Wu KC, Rade JJ, Bivalacqua TJ, Champion HC. Neurohumoral features of myocardial stunning due to sudden emotional stress. N Engl J Med. 2005;352:539–548. doi: 10.1056/NEJMoa043046. [DOI] [PubMed] [Google Scholar]
- Yoshiuchi K, Nomura S, Ando K, Ohtake T, Shimosawa T, Kumano H, Kuboki T, Suematsu H, Fujita T. Hemodynamic and endocrine responsiveness to mental arithmetic task and mirror drawing test in patients with essential hypertension. Am J Hypertens. 1997;10:243–249. doi: 10.1016/s0895-7061(96)00382-2. [DOI] [PubMed] [Google Scholar]
- Young JB, Landsberg L. Sympathoadrenal activity in fasting pregnant rats: Dissociation of adrenal medullary and sympathetic nervous system responses. J Clin Invest. 1979;64:109–116. doi: 10.1172/JCI109429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young JB, Rosa RM, Landsberg L. Dissociation of sympathetic nervous system and adrenal medullary responses. Am J Physiol. 1984;247:E35–E40. doi: 10.1152/ajpendo.1984.247.1.E35. [DOI] [PubMed] [Google Scholar]