Abstract
We report on a family with occipital horn syndrome (OHS) diagnosed in the proband's late fifties. A novel ATP7A pathogenic variant (c.4222A > T, p.(Lys1408*)), representing the first nonsense variant and the second late truncation causing OHS rather than classic Menkes disease, was found to segregate in the family. The predicted maintenance of transmembrane domains is consistent with a residual protein activity, which may explain the mild clinical presentation.
Keywords: Copper transport, Continuum spectrum disorders, ATP7A late truncation, Limited elbow and shoulder movement, Weak grip
1. Introduction
Occipital horn syndrome (OHS, OMIM #304150) takes its name from the pathognomonic occipital horn exostoses. It presents in early to middle childhood and is characterized by connective tissue and skeletal manifestations [1], [2]. Dysautonomia and subtle cognitive deficits might be displayed by some OHS patients; neurological manifestations are predominant in the allelic classic Menkes disease (MD, OMIM #309400). Both disorders, which represent the ends of a continuum, display X-linked recessive inheritance and are caused by pathogenic variants in the ATP7A gene, which codes for a transmembrane copper-transporting ATPase that cycles between the trans‑Golgi (delivery of copper to the secreted copper enzymes) and the plasma (export of surplus intracellular copper) membranes. The phenotypes are mainly related to a deficient activity of cuproenzymes [2], [3]. OHS and mild MD are usually the consequences of pathogenic variants that would result in a protein with residual activity, such as splice-site variants which also produce small amounts of normal protein [4], [5], [6], [7], [8], [9], [10]. Truncating variants, such as exon deletions or nonsense variants, result instead in MD severe classical form with death in early childhood [7], [8]. In addition, a small number of ATP7A missense variants has been recently shown to cause adult-onset isolated distal motor neuropathy, which represents a third distinct phenotype. This neuropathy is mainly caused by subtle defects in ATP7A intracellular trafficking, resulting in preferential localization of the protein at the plasma membrane [11]. We describe a family with OHS first diagnosed in the proband's late fifties. A novel nonsense pathogenic variant in the ATP7A gene was found to segregate in the family.
2. Materials and methods
2.1. Clinical report
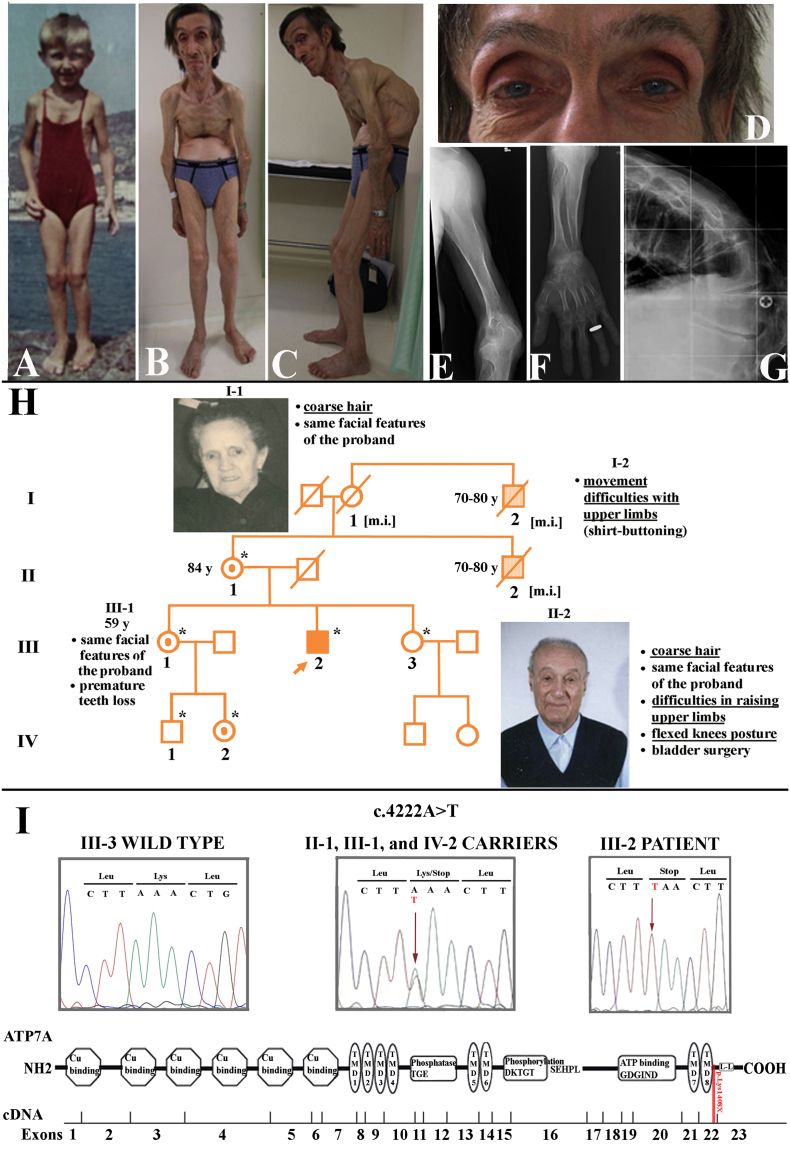
The patient's medical history started at the age of 5 years (Fig. 1A), when he required bladder catheterisation, due to bladder diverticula. At the age of 47 years he had a 20 cm decrement in height with a forward spine curvature (Fig. 1B, C), causing shortening of breath, feeding difficulties, worsening of walking, and urinary disturbances. Free of catheterisations from the age of 10 years until that of 20, he suffered from neurogenic bladder requiring three to four catheterisations a day. As a heavy smoker, he also had chronic obstructive pulmonary disease. Colonic diverticula were diagnosed at the age of 52. Premature teeth loss and surgery for a left inguinal hernia complete his clinical history. Physical examination at 57 years showed the features displayed in Fig. 1B–D, as well as limited extension at the elbows, loose palmar skin, hyperextensible interphalangeal joints of the hands, and dystrophic nails. Head circumference was 57 cm (90th p), height 150 cm.
Fig. 1.
Proband's evolution of the phenotype, his pedigree and family history, and genetic data. A. Photograph of the proband at the age of 4–5 years, documenting that pectus excavatum, flexion of elbows and knees were already manifested. He used to wear a female swimming suit to cover pectus excavatum. B, C. Long thin face, high forehead, hooked nose, short clavicles, severe angled kyphosis, bony outgrowths at the elbows, absence of subcutaneous fat and reduction of muscle mass represent the current phenotype. D. Detail of the proband's face showing prominent orbital arches, iris depigmentation and coarse hair. E–G. Proband's radiographs of the left elbow, forearm and column, showing: (E–G) diffuse severe osteoporosis; (E, F) the exostoses at the head of ulna and radius and its dislocation; (F) metaphyseal flaring and dyaphiseal wavy contours of long bones; (G) wedge-shaped D11 vertebrae, representing the fulcrum of his severe-angled kyphosis, and L1 vertebrae. H. Filled symbol and symbols filled in lighter color represent, respectively, the proband and his two possibly more mildly affected male relatives (see the details in the clinical report). Female carriers of the pathogenic variant are shown with a dot inside the symbol; asterisk, members genotyped in the study; [m.i.], pathogenic variant inferred; y, years. I. From top to bottom: electropherograms of the ATP7A gene sequence of wild type (III-3), carriers (II-1, III-1, and IV-2), and the proband (III-2); schematic view of ATP7A cDNA and protein structure showing in red the approximative position of the pathogenic variant (modified from [24]). (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
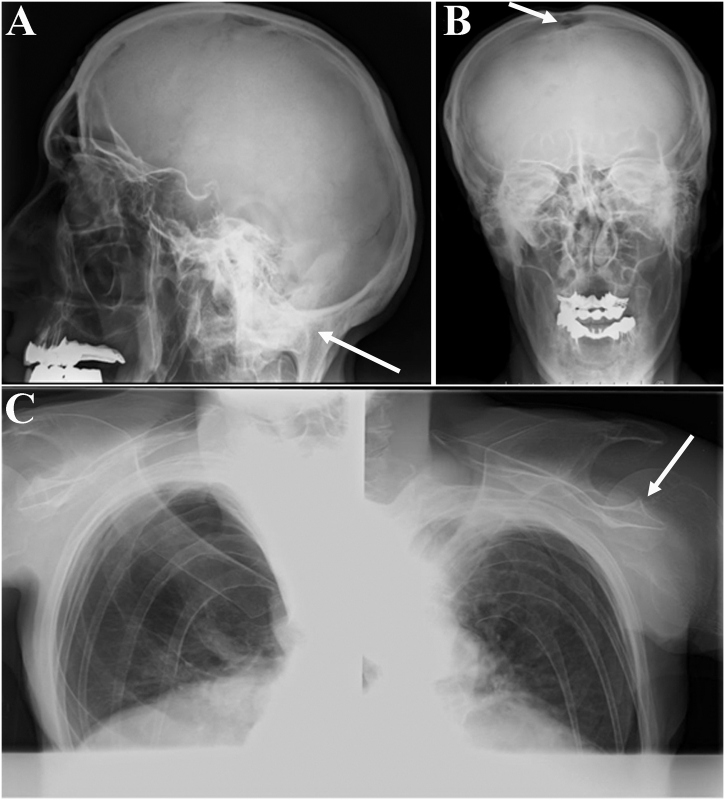
Radiographs of the skeleton demonstrated generalized severe osteoporosis (Fig. 1E–G), that contrasted with the occipital horn exostoses (Supplementary Fig. S1A) and the hyperostosis of the proximal ulna and radius (Fig. 1E, F). Glenohumeral joint alteration (Fig. S1C), together with focal hyperostosis of the elbows and dislocation of the head of the radius into the olecranon (Fig. 1E, F), may explain the patient's inability to touch his head with his hands and the weak grip, which have been present since childhood. The finding of an open anterior fontanel fitted with a sensation of brain splitting (Fig. S1B). Neuroimaging was precluded by his inability to enter scanners. The family pedigree is shown in Fig. 1H.
Supplementary Fig. S1.
Radiographs of the proband's skeleton demonstrated (A) pathognomonic symmetric occipital horn exostoses (arrow), (B) open anterior fontanel (arrow), and (C) flared metaphyses at the head of the humerus and hammer-shaped distal ends of the clavicles (arrow).
Informed consent was obtained from all participants; the study was approved by the Research Ethics Committee of IRCCS Istituto Auxologico Italiano.
2.2. Molecular analysis
ATP7A exons and intron–exon junctions (GenBank accession no. NM_000052.6, GRCh37/hg19) were analysed by Sanger sequencing in the proband's DNA extracted from peripheral blood. Available relatives were genotyped using exon 22-targeted primers. Variant in silico analysis was performed by consulting: Ensembl Genome Browser [12], Single Nucleotide Polymorphism database (dbSNP) [13], and Leiden Open Variation Database (LOVD) ATP7A [14].
3. Results
Blood chemistries demonstrated in the proband low serum levels of copper (40 μg/dL, reference 70–140) and ceruloplasmin (12 mg/dL, reference 20–60). ATP7A sequencing revealed a novel nonsense pathogenic variant in exon 22 (c.4222A > T, p.(Lys1408*)) (Fig. 1I), which was never reported in either healthy subjects or MD/OHS patients. The patient's mother (II-1), the eldest of his two sisters (III-1), and her daughter (IV-2) were found to carry the pathogenic variant (Fig. 1H, I).
4. Discussion
We have described a cognitively normal OHS male, being much older than most OHS or mild MD patients reported in literature [3], [14]. The most disabling features were provoked by severe osteoporosis. Inheritance of the pathogenic variant from the patient's mother (Fig. 1H, II-1) suggests that his maternal uncle (II-2) and granduncle (I-2) could also be affected by OHS. Indeed, they both experienced limited movements of shoulders/upper limbs that suggest the presence of the typical OHS skeletal findings described in the proband (Fig. S1). Moreover, the reported presence of coarse hair in the maternal uncle (II-2) and grandmother (I-1) [10], a possible obligate carrier of the pathogenic variant, further supports this hypothesis.
All the reported ATP7A truncating variants, either early or late [8], [14], [15], cause the classic/severe-classic form of MD, except for the pathogenic variant described by Dagenais et al. [16] and that reported here. Specifically, the OHS family described by Dagenais et al. [16] was found to carry a frameshift variant (c.4352delG) at codon 1451 in exon 23, which gave rise to 13 novel amino acids before a premature stop codon. Apart from the low-average range IQ, the proband's phenotype seems to overlap that of the present patient. In addition, as a result of late truncating variants, both ATP7A transcripts are predicted to lack the distal di–leucine motif, which is considered essential for the protein recycling between the trans-Golgi and the cell membranes. Accordingly, Dagenais et al. [16] demonstrated in their patient abundant ATP7A transcript levels but reduced levels of the corresponding truncated protein, which they proposed to be predominantly located in the plasma membrane. The phenotype was considered as consistent with lysyl oxidase (LOX) – which is involved in collagen and elastin cross-linking – being more sensitive than other cuproenzymes to copper deficiency [16], as LOX needs copper during its synthesis in the endoplasmic reticulum.
Furthermore, by restricting the review to ATP7A truncating variants affecting either exon 22 or 23 [7], [8], [14], [17], [18], [19], [20], the present novel pathogenic variant – as well as that of Dagenais et al. [16] – differs from the other truncations as it is predicted to preserve the eighth transmembrane domain (TMD8), introducing a stop codon just downstream of the predicted TMD8 (Fig. 1I). Conversely, a frameshift variant at position 1400, just 8 codons before the variant here described, but affecting the TMD8, causes a classic MD [20]. This is consistent with the fact that the structure of ATP7A transmembrane domains are extremely conserved and even mild alterations are known to lead to marked reductions in protein activity [8].
5. Conclusion
To our knowledge, the present report is the first to display a nonsense variation resulting in an ATP7A late truncation associated with OHS rather than MD. The patient's phenotype suggests that ATP7A retains a certain degree of residual activity when TMD8 remains intact, despite the absence of the C-terminal 93 amino acids. The limit of truncating variants with residual enzymatic activity can therefore be shifted from exon 23 (codon 1451) to exon 22 (codon 1408). Moreover, the patient's possibly affected male relatives further support the idea that the intrafamilial phenotypic variability might be very wide when the variant has a residual enzymatic activity [4], [21], [22], [23], and may be due to different expression of the mutant ATP7A, as previously demonstrated [23]. As a consequence of OHS phenotypic variability, males with ID and connective tissue abnormalities should be evaluated for biochemical evidence of defective copper transport.
The following is the supplementary data related to this article.
Acknowledgements
The authors would like to thank the family members for their collaboration. We also thank Prof. Fiorenza Bellini (Radiology Unit, IRCCS Istituto Auxologico Italiano, Milan) for helping in the description and interpretation of skeletal findings.
Acknowledgments
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Conflict of interests
None to declare.
Contributor Information
Maria Teresa Bonati, Email: mt.bonati@auxologico.it.
Federico Verde, Email: fdrc.verde@unimi.it.
Uros Hladnik, Email: Uros.Hladnik@birdfoundation.org.
Paola Cattelan, Email: cattelanp@libero.it.
Luca Campana, Email: l.campana@auxologico.it.
Chiara Castronovo, Email: c.castronovo@auxologico.it.
Nicola Ticozzi, Email: n.ticozzi@auxologico.it.
Luca Maderna, Email: l.maderna@auxologico.it.
Claudia Colombrita, Email: claudiacolombrita@hotmail.com.
Sergio Papa, Email: sergio.papa@cdi.it.
Paolo Banfi, Email: pabanfi@dongnocchi.it.
Vincenzo Silani, Email: vincenzo@silani.com.
References
- 1.Sartoris D.J., Luzzatti L., Weaver D.D., Macfarlane J.D., Hollister D.W., Parker B.R. Type IX Ehlers-Danlos syndrome. A new variant with pathognomonic radiographic features. Radiology. 1984;152:665–670. doi: 10.1148/radiology.152.3.6463246. [DOI] [PubMed] [Google Scholar]
- 2.Horn N., Tümer Z. Menkes disease and the occipital horn syndrome. In: Royce P.M., Steinman B., editors. Connective Tissue and Its Heritable Disorders. Wiley-Liss, Inc.; New York: 2002. pp. 651–685. [Google Scholar]
- 3.Tümer Z., Møller L.B. Menkes disease. Eur. J. Hum. Genet. 2010;18:511–518. doi: 10.1038/ejhg.2009.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kaler S.G., Gallo L.K., Proud V.K., Percy A.K., Mark Y., Segal N.A. Occipital horn syndrome and a mild Menkes phenotype associated with splice site mutations at the MNK locus. Nat. Genet. 1994;8:195–202. doi: 10.1038/ng1094-195. [DOI] [PubMed] [Google Scholar]
- 5.Das S., Levinson B., Vulpe C., Whitney S., Gitschier J., Packman S. Similar splicing mutations of the Menkes/mottled copper-transporting ATPase gene in occipital horn syndrome and the blotchy mouse. Am. J. Hum. Genet. 1995;56:570–576. [PMC free article] [PubMed] [Google Scholar]
- 6.Levinson B., Conant R., Schnur R., Das S., Packman S., Gitschier J. A repeated element in the regulatory region of the MNK gene and its deletion in a patient with occipital horn syndrome. Hum. Mol. Genet. 1996;5:1737–1742. doi: 10.1093/hmg/5.11.1737. [DOI] [PubMed] [Google Scholar]
- 7.Skjørringe T., Tümer Z., Møller L.B. Splice site mutations in the ATP7A gene. PLoS One. 2011;6 doi: 10.1371/journal.pone.0018599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tümer Z. An overview and update of ATP7A mutations leading to Menkes disease and occipital horn syndrome. Hum. Mutat. 2013;34:417–429. doi: 10.1002/humu.22266. [DOI] [PubMed] [Google Scholar]
- 9.Møller L.B., Tümer Z., Lund C., Petersen C., Cole T., Hanusch R. Similar splice-site mutations of the ATP7A gene lead to different phenotypes: classical Menkes disease or occipital horn syndrome. Am. J. Hum. Genet. 2000;66:1211–1220. doi: 10.1086/302857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Møller L.B. Small amounts of functional ATP7A protein permit mild phenotype. J. Trace Elem. Med. Biol. 2015;31:173–177. doi: 10.1016/j.jtemb.2014.07.022. [DOI] [PubMed] [Google Scholar]
- 11.Kennerson M.L., Nicholson G.A., Kaler S.G., Kowalski B., Mercer J.F., Tang J. Missense mutations in the copper transporter gene ATP7A cause X-linked distal hereditary motor neuropathy. Am. J. Hum. Genet. 2010;86:343–352. doi: 10.1016/j.ajhg.2010.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ensembl. http://www.ensembl.org (accessed 04.01.2017)
- 13.dbSNP. https://www.ncbi.nlm.nih.gov/projects/SNP/ (accessed 04.01.2017)
- 14.LOVD ATP7A. https://grenada.lumc.nl/LOVD2/MD/home.php (accessed 04.01.2017)
- 15.Lin Y.J., Ho C.S., Hsu C.H., Lin J.L., Chuang C.K., Tsai J.D. A truncating de novo point mutation in a young infant with severe Menkes disease. Pediatr. Neonatol. 2017;58:89–92. doi: 10.1016/j.pedneo.2014.05.008. [DOI] [PubMed] [Google Scholar]
- 16.Dagenais S.L., Adam A.N., Innis J.W., Glover T.W. A novel frameshift mutation in exon 23 of ATP7A (MNK) results in occipital horn syndrome and not in Menkes disease. Am. J. Hum. Genet. 2001;69:420–427. doi: 10.1086/321290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gu Y.H., Kodama H., Murata Y., Mochizuki D., Yanagawa Y., Ushijima H. ATP7A gene mutations in 16 patients with Menkes disease and a patient with occipital horn syndrome. Am. J. Med. Genet. 2001;99:217–222. doi: 10.1002/1096-8628(2001)9999:9999<::aid-ajmg1167>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 18.Kaler S.G., Holmes C.S., Goldstein D.S., Tang J., Godwin S.C., Donsante A. Neonatal diagnosis and treatment of Menkes disease. N. Engl. J. Med. 2008;358:605–614. doi: 10.1056/NEJMoa070613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kaler S.G., Liew C.J., Donsante A., Hicks J.D., Sato S., Greenfield J.C. Molecular correlates of epilepsy in early diagnosed and treated Menkes disease. J. Inherit. Metab. Dis. 2010;33:583–589. doi: 10.1007/s10545-010-9118-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tümer Z., Lund C., Tolshave J., Vural B., Tonnesen T., Horn N. Identification of point mutations in 41 unrelated patients affected with Menkes disease. Am. J. Hum. Genet. 1997;60:63–71. [PMC free article] [PubMed] [Google Scholar]
- 21.Kaler S.G. Menkes Disease. Adv. Pediatr. Infect. Dis. 1994;41:263–304. [PubMed] [Google Scholar]
- 22.Borm B., Møller L.B., Hausser I., Emeis M., Baerlocher K., Horn N. Variable clinical expression of an identical mutation in the ATP7A gene for Menkes disease/occipital horn syndrome in three affected males in a single family. J. Pediatr. 2004;145:119–121. doi: 10.1016/j.jpeds.2004.04.033. [DOI] [PubMed] [Google Scholar]
- 23.Donsante A., Tang J., Godwin S.C., Holmes C.S., Goldstein D.S., Bassuk A. Differences in ATP7A gene expression underlie intrafamilial variability in Menkes disease/occipital horn syndrome. J. Med. Genet. 2007;44:492–497. doi: 10.1136/jmg.2007.050013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Moizard M.P., Ronce N., Blesson S., Bieth E., Burglen L., Mignot C. Twenty-five novel mutations including duplications in the ATP7A gene. Clin. Genet. 2011;79:243–253. doi: 10.1111/j.1399-0004.2010.01461.x. [DOI] [PubMed] [Google Scholar]