Figure 4.
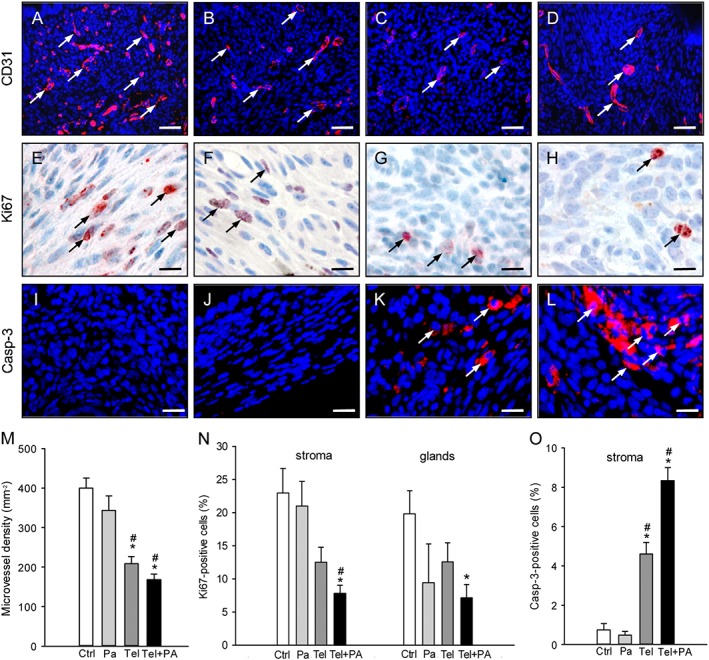
Immunohistochemical analysis of vascularization, cell proliferation and apoptotic cell death within endometriotic lesions. Immunofluorescent detection of microvessels (arrows) within endometriotic lesions on day 28 after surgical induction by fixation of uterine tissue samples to the abdominal wall of a vehicle‐treated control (A) as well as a parecoxib‐ (B), a telmisartan (C) and a parecoxib/telmisartan‐ (D) treated C57BL/6 mouse. Sections were stained with Hoechst 33342 to identify cell nuclei (blue) and an antibody against CD31 (red) for the detection of microvessels. Scale bars: 50 μm. Immunohistochemical detection of proliferating Ki67‐positive cells (arrows) in the stroma of endometriotic lesions of a vehicle‐treated control (E) as well as of a parecoxib‐ (F), a telmisartan‐ (G) and a parecoxib/telmisartan‐ (H) treated C57BL/6 mouse. Scale bars: 15 μm. Immunofluorescent detection of apoptotic cells (arrows) within endometriotic lesions of a vehicle‐treated control (I) as well as a parecoxib‐ (J), a telmisartan (K) and a parecoxib/telmisartan‐ (L) treated C57BL/6 mouse. Sections were stained with Hoechst 33342 to identify cell nuclei (blue) and an antibody against cleaved caspase‐3 (casp‐3; red) for the detection of apoptotic cells. Scale bars: 20 μm. Microvessel density (M, mm−2), Ki67‐positive cells (N, %) and cleaved caspase‐3‐positive cells (O, %) of endometriotic lesions of vehicle‐treated controls (Ctrl), parecoxib‐ (Pa), telmisartan‐ (Tel) and parecoxib/telmisartan‐ (Tel+PA) treated C57BL/6 mice. Means ± SEM (n = 10 for each experimental group). *P < 0.05 versus control; # P < 0.05 versus parecoxib.
