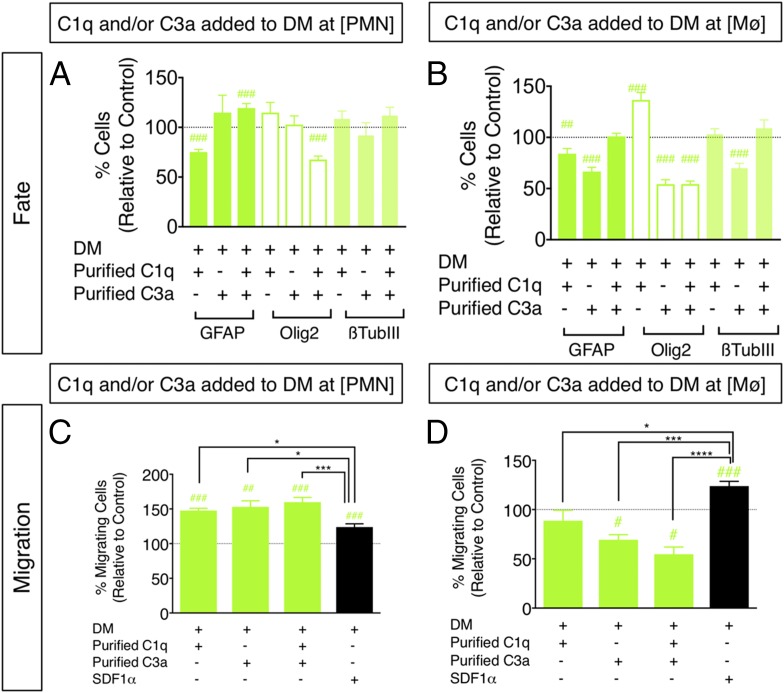
FIGURE 6.
Treatment of hNSC with purified C1q and C3a modulates hNSC fate and migration in vitro. (A and B) hNSC were treated with purified C1q and/or C3a at [PMN] or [Mϕ], followed by immunocytochemical quantification of GFAP+, nuclear Olig2+, and β-tubulin III+ cell number. Comparisons versus DM control (dashed line) were conducted using one-sample t tests (#p < 0.05, ##p < 0.01, ###p < 0.001). hNSC differentiation in the presence of C1q plus C3a[PMN], but not C1q plus C3a[Mϕ] increased the percentage of GFAP+ cells and decreased the percentage of Olig2+ cells at 14 DIV compared with DM control. (C and D) hNSC were treated with purified C1q and/or C3a at [PMN] or [Mϕ], followed by Transwell assay quantification of migration, respectively. Comparisons versus DM control (dashed line) were conducted using one-sample t tests (#p < 0.05, ##p < 0.01, ###p < 0.001). Comparisons across multiple groups were conducted using one-way ANOVA [(C): F = 8.513, p < 0.0005; (D): F = 18.84, p < 0.0001], followed by Tukey post hoc tests (*p < 0.05, ***p < 0.001, ****p < 0.0001). Addition of C1q[PMN], C3a[PMN], and C1q plus C3a[PMN] increased hNSC migration versus DM control, and relative to SDF1α. Addition of C3a[Mϕ] and C1q plus C3a[Mϕ] decreased hNSC migration versus DM control, and relative to SDF1α. n = 2 technical replicates and n = 3–4 biological replicates/condition. Mean ± SEM.