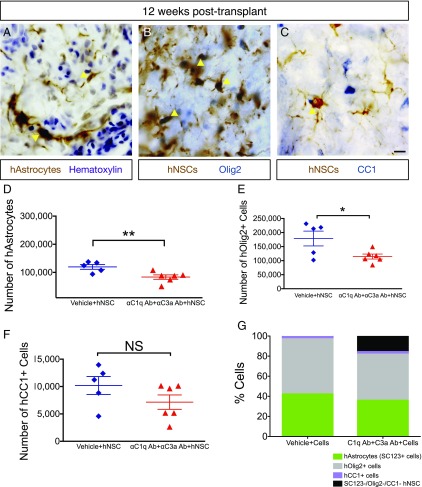
FIGURE 10.
αC1q Ab plus αC3a Ab injection reduces glial differentiation in vivo. Twelve week posttransplant, mice were transcardially perfused and tissue collected for histology. (A) Immunohistochemistry in transverse spinal cord sections for the human specific astrocytic marker, SC123 (brown); hematoxylin counterstain (purple) at 12 wk posttransplant. Yellow arrowheads indicate human astrocytes (hAstrocytes) that were quantified for unbiased stereology in (D). (B) Immunohistochemistry in transverse spinal cord sections for the human cytoplasmic marker, SC121 (brown); oligodendroglial progenitors were identified using nuclear Olig2 (blue) at 12 wk posttransplant. Yellow arrowheads indicate human Olig2+ cells (hOlig2) that were quantified for unbiased stereology in (E). (C) Immunohistochemistry in transverse spinal cord sections for the human cytoplasmic marker, SC121 (brown); mature oligodendroglial marker, CC1 (blue), at 12 wk posttransplant. Yellow arrowheads indicate human CC1+ cells (hCC1) that were quantified for unbiased stereology in (F). (D–F) At 12 wk posttransplant, fate of hNSC was quantified using unbiased stereology and the optical fractionator probe in SCI mice that received vehicle plus hNSC (blue diamonds) and those receiving αC1q plus αC3a Abs plus hNSC (red triangles). Comparisons were conducted using Student t tests (NS is p > 0.05, *p < 0.05, **p < 0.01). (D and E) In the presence of αC1q plus αC3a Abs, the number of hAstrocytes (D) and hOlig2+ cells (E) significantly decreased compared with control (vehicle plus hNSC), but the number of hCC1+ cells remained unaltered as a result of Ab injection (F). (G) In mice receiving vehicle plus hNSC, hAstrocytes, hOlig2+, and hCC1+ cells accounted for 100% engrafted hNSC; in contrast, in mice treated with αC1q plus αC3a Abs, these markers accounted for only 84% engrafted hNSC. Mean ± SEM is shown. Scale bar, 25 μm.