Figure 1.
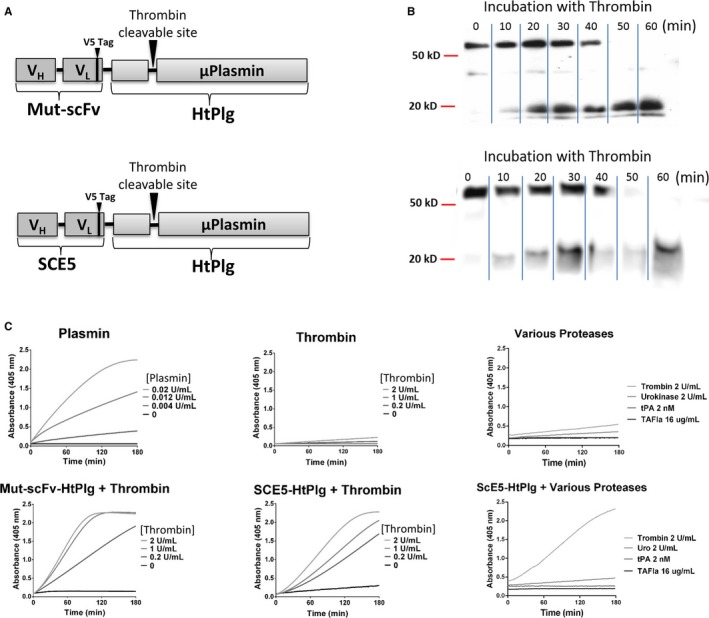
A, Schematic representation of the anti–glycoprotein (GP)IIb/IIIa single‐chain antibody (SCE5)–human thrombin‐activatable microplasminogen (HtPlg) and nontargeted control scFv HtPlg (Mut‐scFv‐HtPlg) constructs. The amino acid sequence of the plasminogen activator site from human plasminogen was substituted for the thrombin cleavage site from factor XIII. The HtPlg construct was then fused with two different single‐chain antibodies, one targeting activated GPIIb/IIIa (SCE5) and the other Mut‐scFv. B, Cleavage and generation of microplasmin after thrombin incubation was demonstrated in vitro. The Mut‐scFv‐HtPlg or the SCE5‐HtPlg (200 μg/mL) was incubated at 37°C with thrombin (3 U/mL) and samples were withdrawn at 0, 10, 20, 30, 40, 50, and 60 minutes, mixed with dithiothreitol and analyzed on Western blots using horseradish peroxidase coupled to an anti‐V5 antibody. C, The activation of the SCE5‐HtPlg (13 μg/mL) and of the Mut‐scFv‐HtPlg (13 μg/mL) to microplasmin after incubation with different thrombin concentrations (0, 0.2, 1, and 2 U/mL) was demonstrated. The SCE5‐HtPlg was additionally tested with urokinase (2 U/mL), tPA (2 nmol/L), and thrombin‐activatable fibrinolysis inhibitor (TAFIa) (16 μg/mL). Microplasmin generation was monitored over 2 hours by spectrophotometry at 405 nm with the plasmin chromogenic substrate S2251 (350 μmol/L). Positive control was obtained with different plasmin concentrations (0, 0.004, 0.012, and 0.02 U/mL) and negative control was obtained with only thrombin (0, 0.2, 1, and 2 U/mL).
