Abstract
Background
Impaired left atrial (LA) mechanical function is present in hypertension and likely contributes to various complications, including atrial arrhythmias, stroke, and heart failure. Various antihypertensive drug classes exert differential effects on central hemodynamics and left ventricular function. However, little is known about their effects on LA function.
Methods and Results
We studied 212 subjects with hypertension and without heart failure or atrial fibrillation. LA strain was measured from cine steady‐state free‐precession cardiac MRI images using feature‐tracking algorithms. In multivariable models adjusted for age, sex, race, body mass index, blood pressure, diabetes mellitus, LA volume, left ventricular mass, and left ventricular ejection fraction, beta‐blocker use was associated with a lower total longitudinal strain (standardized β=−0.21; P=0.008), and lower LA expansion index (standardized β=−0.30; P<0.001), indicating impaired LA reservoir function. Beta‐blocker use was also associated with a lower positive strain (standardized β=−0.19; P=0.012) and early diastolic strain rate (standardized β=0.15; P=0.039), indicating impaired LA conduit function. Finally, beta‐blocker use was associated with a lower (less negative) late‐diastolic strain (standardized β=0.15; P=0.049), strain rate (standardized β=0.18; P=0.019), and a lower active LA emptying fraction (standardized β=−0.27; P<0.001), indicating impaired booster pump function. Use of other antihypertensive agents was not associated with LA function.
Conclusions
Beta‐blocker use is significantly associated with impaired LA function in hypertension. This association could underlie the increased risk of atrial fibrillation and stroke seen with the use of beta‐blockers (as opposed to other antihypertensive agents) demonstrated in recent trials.
Keywords: angiotensin‐converting enzyme inhibitors, β‐adrenergic antagonists, hypertension, left atrium, magnetic resonance imaging
Subject Categories: Hypertension, Magnetic Resonance Imaging (MRI), Atrial Fibrillation
Introduction
An increase in left atrial (LA) size in patients with hypertension has long been known to be associated with worse cardiovascular morbidity and mortality.1, 2 Over the last decade, however, the prognostic importance of phasic LA function, independent of LA size, has been recognized.3 Compensatory changes in dynamic LA function are seen early in the course of hypertension, even before left ventricular hypertrophy (LVH) develops. Early in response to the impaired left ventricular (LV) relaxation in hypertension, the conduit function of the LA decreases, whereas its booster function increases.4 However, with progression of hypertensive heart disease, booster function also declines and is associated with progression to heart failure5, 6 and a poor prognosis.7
Various classes of antihypertensive medications exert differential effects on LV function and central hemodynamics. Available trials, including the Losartan Intervention For Endpoint reduction (LIFE) and the Anglo Scandinavian Cardiac Outcomes Trial (ASCOT) [as well as the Conduit Artery Function Evaluation (CAFE) substudy of ASCOT] have shown that, among patients with hypertension, atenolol, when compared to angiotensin receptor blockers (ARBs) and calcium‐channel blockers, is associated with lower LV mass regression,8 greater central systolic pulse pressure relative to brachial pressures (ie, lower pulse pressure amplification),9 and a higher risk of stroke10 and atrial fibrillation (AF).11 Unfortunately, little is known about the effects of various antihypertensive agents on LA function. In particular, an unfavorable effect of beta‐blockers on LA function is a plausible mechanism for the increased risk of AF and stroke associated with beta‐blocker use, compared to angiotensin receptor blockade.11 However, no data are available regarding the association between the use of beta‐blockers (or other antihypertensive drugs) and LA function.
In this study we aimed to assess whether the use of beta‐blockers, when compared to other antihypertensive agents, is associated with impaired LA function, as assessed by MRI‐based measurements of atrial deformation and phasic volumes.
Methods
Study Population
We enrolled a convenience clinical sample of 212 subjects with history of hypertension at the Corporal Michael J. Crescenz VA Medical Center. The protocol was approved by the Philadelphia VA Medical Center Institutional Review Board, and written informed consent was obtained from all participants. Key exclusion criteria were as follows: (1) claustrophobia; (2) presence of metallic objects or implanted medical devices within the body; (3) AF at the time of enrollment; (4) conditions that would make the study measurements less accurate or unreliable (ie, arrhythmia affecting cardiac gating, inability to perform an adequate breath hold for cardiac MRI acquisitions); (5) heart failure with preserved or reduced ejection fraction (EF); and (6) a left ventricular EF <50%.
Cine Imaging
Participants underwent a cardiac MRI examination to assess LV structure and function, using a 1.5‐Tesla (T) whole‐body MRI scanner (Avanto or Espree, Siemens, Malvern, PA) equipped with a phased‐array cardiac coil. LV volumes and EF were determined using balanced steady‐state free‐precession (SSFP) cine imaging. Typical parameters were as follows: TR=30.6 milliseconds; TE=1.3 milliseconds; phases=30; slice thickness=8 mm; matrix size=192×192; and parallel image (IPAT) factor=2. LV short‐axis stack cine images were manually traced at end‐diastole and end‐systole using CMR42 software (Circle CVI, Calgary, AB, Canada). LV mass (LVM) was computed as the difference between epicardial and endocardial volumes, multiplied by myocardial density. LVM was normalized for body height in meters raised to the allometric power of 1.7.12
Left Atrial Longitudinal Strain and Volumetric Analysis
All image analyses were performed using cvi42 image analysis software (Circle Cardiovascular Imaging Inc, Calgary, AB, Canada), blinded to the clinical characteristics of the study subjects. Feature‐tracking techniques for the measurement of LA phasic strain have previously been described.13, 14 LA endocardial borders were manually traced in apical 2‐ and 4‐chamber views using LV end‐diastole as the point of reference. An automated tracking algorithm was applied, and the tracking of all atrial segments was confirmed. Manual adjustments were performed as needed to optimize wall tracking. An example of atrial wall tracking is shown in Figure 1 and in Video S1.
Figure 1.
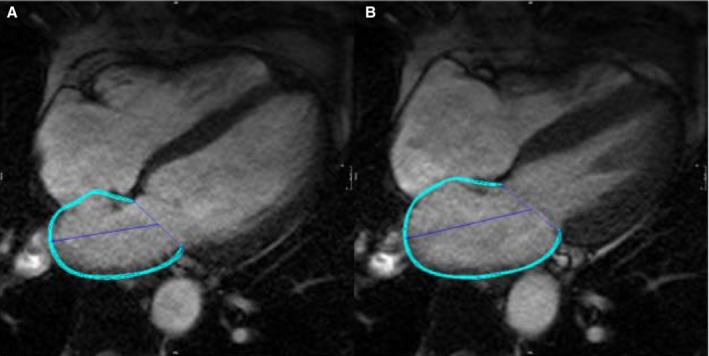
Representative example of atrial tissue tracking using cine SSFP‐MRI images. A, The diastatic left atrial phase, in which reference points are prescribed. B, The tracking of atrial tissue, shown at a different phase of the cardiac cycle (see Video S1). The blue line represents the atrial wall contour. MRI indicates magnetic resonance imaging; SSFP, steady‐state free‐precession.
Values of segmental deformation were exported and further processed using custom software programmed in Python (Python Software Foundation, Wilmington, DE). We recomputed strain (deformation) and strain rate relative to the diastatic LA length (rather than end‐diastolic length), because diastasis represents the length for LA tissue at the end of atrial diastole (reference phase). As shown in Figure 2, strain and strain rate were calculated in the reservoir (total longitudinal strain), conduit (positive longitudinal strain and early‐diastolic strain rate), and booster (negative longitudinal strain and late‐diastolic strain rate) phases.14 Maximum (LAMAX), minimum (LAMIN), and diastatic (LADIAS) LA volumes were also measured. LA expansion index, passive LA emptying fraction (LAEF), and active LAEF were calculated as volumetric measures of reservoir, conduit, and booster phases, respectively. LA expansion index was calculated as (LAMAX−LAMIN)/LAMIN, passive LAEF as (LAMAX−LADIAS)/LAMAX, and active LAEF as (LADIAS−LAMIN)/LAMAX.
Figure 2.
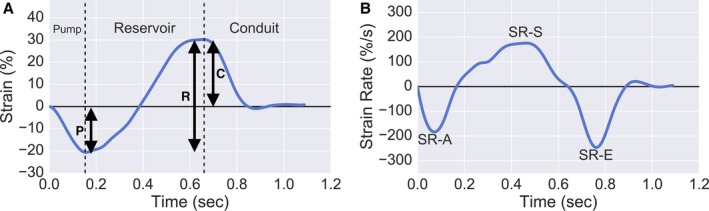
Representative Plots of Strain (A), and Strain rate (B), along with measures of reservoir, conduit and booster pump function derived from strain and strain rate measurements. A, left atrial strain curve, where total (reservoir, R), positive (conduit, C) and negative (booster pump, P) strain are demonstrated. B, left atrial strain rate curve, where early diastolic (SR‐E), late‐diastolic (SR‐A) and systolic (SR‐S) strain rate can be demonstrated. SR‐E and SR‐A represent left atrial conduit and booster pump function, respectively. Note that atrial diastasis was used as the reference length for all strain/strain rate measurements.
Measurement of Central Blood Pressure
Arterial tonometry of the common carotid artery was performed using a SphygmoCor EM3 device (AtCor Medical, Sydney, Australia), equipped with a high‐fidelity Millar Applanation tonometer (SPT‐301; Millar Instruments, Houston, TX). Tonometry was performed at the carotid artery in the supine position immediately after the MRI. The carotid waveform was calibrated with brachial diastolic and mean blood pressures (which do not vary substantially along the arterial tree) and were used as a direct surrogate of the central pressure waveform. This approach does not require the use of a generalized transfer function.
Statistical Methods
Continuous variables are presented as mean±SD. Categorical variables are presented as frequencies and percentages. Various general characteristics and parameters of LA function were compared between subjects who were receiving beta‐blockers versus those who were not. For continuous variables, we used nonpaired t tests or Wilcoxon signed‐rank tests as appropriate. For categorical variables, we used chi‐squared tests. Multivariable linear regression was then performed to determine the relationship between beta‐blocker use (expressed as a binary variable) and various measures of LA function. This relationship was assessed after adjustment for potential confounders, including age, sex, race, body mass index, systolic blood pressure, diastolic blood pressure, diabetes mellitus, LA volume, LV mass, and LV EF. For easier comparisons of the magnitude of the relationships of different predictors, we present standardized regression coefficients (β). All probability values are 2‐tailed. Statistical significance was defined as a 2‐tailed P<0.05. All statistical analyses were performed using SPSS software (SPSS v24 for Mac; IBM SPSS version 24, Chicago, IL).
Results
Baseline characteristics of study participants are presented in Table 1. The average age was 63±10 years; the majority of subjects were males (94%), with similar proportions of white and black participants. There was a moderate prevalence of other cardiovascular risk factors, including diabetes mellitus, hyperlipidemia, and smoking. Mean systolic and diastolic blood pressures were 147±19 and 84±12 mm Hg. The majority of participants were receiving more than 1 antihypertensive agent at the time of enrolment. There were no significant differences in most general characteristics between subjects who were receiving beta‐blockers and those who were not, except for a slightly lower prevalence of smoking, a slightly lower LV EF, and a significantly greater prevalence of coronary artery disease among beta‐blocker users. Central hemodynamic data (available in 176 subjects: 89 who used beta‐blockers and 87 who did not) are also presented in Table 1. In contrast to brachial pulse pressure, central pulse pressure tended to be greater in beta‐blocker users (62.9 mm Hg vs 56.6 mm Hg; P=0.065). Central‐to‐brachial pulse pressure amplification was significantly lower in beta‐blocker users (1.08 vs 1.18, P=0.008).
Table 1.
Baseline Characteristics of Study Participants
| Characteristics | Beta‐blocker Use (n=106) | No Beta‐blocker Use (n=106) | P Value |
|---|---|---|---|
| Age, y | 63±9 | 62±10 | 0.41 |
| Male sex | 102 (96) | 97 (91) | 0.15 |
| Race | |||
| White | 49 (46) | 51 (48) | 0.49 |
| Black | 49 (46) | 52 (49) | 0.49 |
| Body mass index, kg/m2 | 31±7 | 31±7 | 0.87 |
| Systolic blood pressure, mm Hg | 148±19 | 145±18 | 0.26 |
| Diastolic blood pressure, mm Hg | 84±14 | 84±11 | 0.46 |
| Mean blood pressure, mm Hg | 117±17 | 114±14 | 0.16 |
| Pulse pressure, mm Hg | 63.8 (13.6) | 62.1 (14.4) | 0.39 |
| Current smoker | 19 (18) | 33 (31) | 0.025 |
| Diabetes mellitus | 47 (44.3) | 53 (50) | 0.41 |
| Hemoglobin A1C, % | 6.5±1.3 | 6.4±1.6 | 0.61 |
| eGFR, mL/(min·1.73 m2) | 85±29 | 86±30 | 0.80 |
| Coronary artery disease | 18 (17) | 45 (42.5) | <0.001 |
| ACE inhibitor use | 62 (59) | 54 (51) | 0.27 |
| ARB use | 13 (12) | 10 (9) | 0.51 |
| Calcium‐channel blocker use | 36 (34) | 39 (37) | 0.67 |
| Thiazide use | 32 (30.2) | 38 (36.2) | 0.36 |
| Spironolactone use | 6 (5.7) | 2 (1.9) | 028 |
| Specific beta‐blocker used | |||
| Metoprolol | 55 (51.9) | — | — |
| Carvedilol | 28 (26.4) | — | — |
| Atenolol | 13 (12.3) | — | — |
| Labetalol | 4 (3.8) | — | — |
| Propranolol | 4 (3.8) | — | — |
| Other | 2 (1.9) | — | — |
| LVEF, % | 60±8 | 62±8 | 0.035 |
| LVM, g | 154±41 | 151±38 | 0.54 |
| LVM index, g/m1.7 | 58±14 | 58±14 | 0.89 |
| Left atrial volume index, mL/m2 | 33±14 | 34±22 | 0.58 |
| E to septal eʹ ratio | 11±6 | 11±8 | 0.65 |
| Resting heart rate, beats/min | 61.9±11.1 | 65.2±11.9 | 0.053 |
| Carotid augmentation indexa | 13.6±15.2 | 11.2±16.7 | 0.12 |
| Carotid pulse pressurea | 62.9±23.5 | 56.6±22.1 | 0.065 |
| Carotid‐to‐brachial pressure amplificationa | 1.08±0.23 | 1.18±0.28 | 0.008 |
Values are mean±SD or counts (percentages). ACE indicates angiotensin‐converting enzyme; ARB, angiotensin receptor blocker; eGFR, estimated glomerular filtration rate; LVEF, left ventricular ejection fraction; LVM, left ventricular mass.
Data available in 176 subjects (89 who used beta‐blockers and 87 who did not use beta‐blockers).
Correlation of Left Atrial Parameters With Antihypertensive Medications
Table 2 shows values of LA deformation measures and volumes among subjects who were receiving beta‐blockers versus those who were not. Total longitudinal strain and LA expansion index were significantly lower in subjects receiving beta‐blockers (total longitudinal strain 24% vs 28%, P=0.005; LA expansion index 1.10 vs 1.35, P<0.001, Figure 3). Similarly, negative longitudinal strain (−13% vs −15%, P=0.008), late‐diastolic strain rate (−140%/s vs −163%/s, P=0.008), and active LAEF (37% vs 42%, P=0.001) were lower in subjects receiving beta‐blockers. Less negative values of late‐diastolic strain and strain rate, present in subjects receiving beta‐blockers, indicate worse LA booster pump function. Subjects receiving beta‐blockers also demonstrated lower values of negative early‐diastolic strain rate (−83%/s vs −99%/s, P=0.022), indicating impaired conduit function.
Table 2.
Left Atrial Parameters Among Subjects Receiving Beta‐blockers and Subjects Not Receiving Beta‐blockers
| Left Atrial Parameters | Beta‐blocker Use | No Beta‐blocker Use | P Value |
|---|---|---|---|
| Reservoir function | |||
| Total longitudinal strain, % | 24±10 | 28±11 | 0.005 |
| LA expansion index | 1.10±0.41 | 1.35±0.43 | <0.001 |
| Conduit function | |||
| Positive longitudinal strain, % | 11±7 | 13±8 | 0.06 |
| Early‐diastolic strain rate, %/sa | −83±49 | −99±54 | 0.022 |
| Passive LA ejection fraction, % | 22±8 | 23±9 | 0.13 |
| Booster pump function | |||
| Negative longitudinal strain, %a | −13±6 | −15±6 | 0.008 |
| Late‐diastolic strain rate, %/sa | −140±62 | −163±66 | 0.008 |
| Active LA emptying fraction, % | 37±10 | 42±10 | 0.001 |
LA indicates left atrium.
Less negative value of diastolic strain and strain rate indicates worse LA function.
Figure 3.
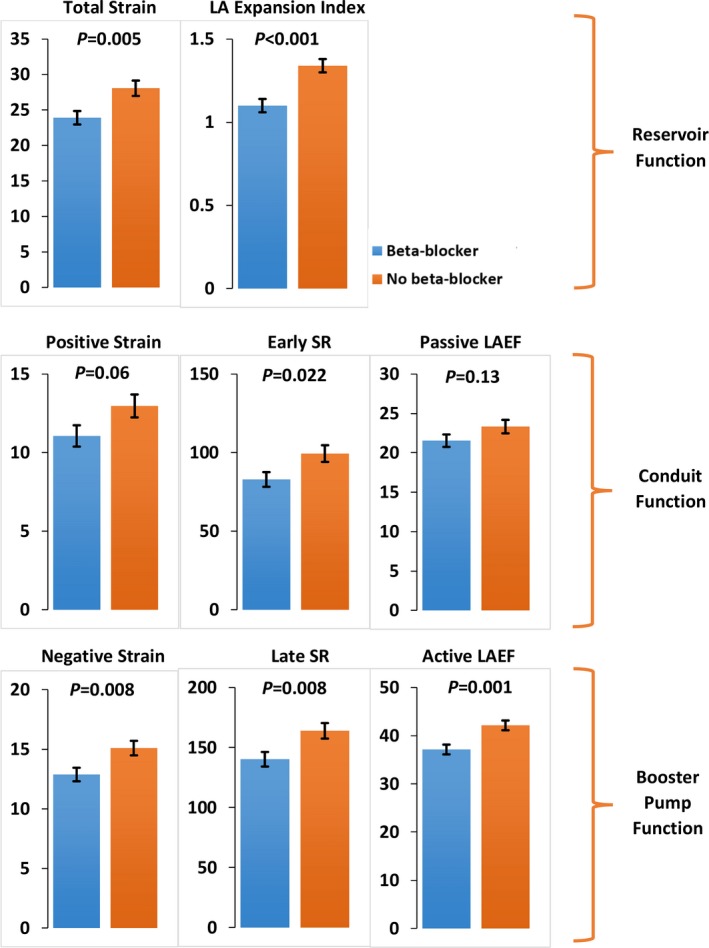
Bar graphs comparing various left atrial parameters in participants on beta‐blockers to participants not on beta‐blockers. LA indicates left atrium; LAEF, left atrial emptying fraction; SR, strain rate. Absolute values for early‐diastolic strain rate, negative longitudinal strain, and late‐diastolic strain rate are presented for the ease of direct comparison between the groups. Error bars represent standard errors of the mean.
Table 3 shows the results of multivariable models in which the relationship between beta‐blocker use and LA function is analyzed. In models that adjusted for adjusted for age, sex, and race (Model 1, Table 3), beta‐blocker use was consistently associated with measures of impaired reservoir and booster pump function, including lower total longitudinal strain, LA expansion index, negative longitudinal strain, late‐diastolic strain rate, and active LAEF. In addition, beta‐blocker use was associated with a lower (less negative) early‐diastolic strain rate (standardized β=0.14: P=0.036) in these adjusted models.
Table 3.
Unadjusted and Adjusted Standardized Regression Coefficients for the Association of Left Atrial Measures With Beta‐blocker Use
| Left Atrial Parameters | Model 1 Estimated Standardized β Coefficient (P Value) | Model 2 Estimated Standardized β Coefficient (P Value) | Model 3 Estimated Standardized β Coefficient (P Value) |
|---|---|---|---|
| Reservoir function | |||
| Total longitudinal strain | −0.18 (0.009) | −0.21 (0.005) | −0.21 (0.006) |
| LA expansion index | −0.26 (<0.001) | −0.27 (<0.001) | −0.30 (<0.001) |
| Conduit function | |||
| Positive longitudinal strain | −0.11 (0.094) | −0.16 (0.024) | −0.19 (0.012) |
| Early‐diastolic strain ratea | 0.14 (0.036) | 0.17 (0.016) | 0.15 (0.039) |
| Passive LA ejection fraction | −0.08 (0.235) | −0.10 (0.140) | −0.08 (0.26) |
| Booster pump function | |||
| Negative longitudinal straina | 0.18 (0.010) | 0.16 (0.028) | 0.15 (0.049) |
| Late‐diastolic strain ratea | 0.18 (0.009) | 0.16 (0.041) | 0.18 (0.019) |
| Active LA emptying fraction | −0.23 (0.001) | −0.26 (0.001) | −0.27 (<0.001) |
Model 1: adjusted for age, sex, and race. Model 2: adjusted for variables in Model 1 plus body mass index, systolic and diastolic blood pressure, presence of diabetes mellitus, coronary artery disease, use of angiotensin‐converting enzyme inhibitors, angiotensin receptor blockers, calcium‐channel blockers, spironolactone, and diuretics. Model 3: adjusted for variables in Model 2 plus LA volume, left ventricular mass, and left ventricular ejection fraction. LA indicates left atrium.
Positive coefficients in diastolic strain and strain rate indicate “less negative values” and therefore worse left atrial function.
In multivariable models that adjusted for age, sex, race, body mass index, blood pressure, the presence of diabetes mellitus, and use of other antihypertensive agents (Model 2, Table 3), beta‐blocker use was associated with lower total longitudinal strain (standardized β=−0.21; P=0.005), and lower LA expansion index (standardized β=−0.27; P<0.0001), indicating impaired LA reservoir function. Beta‐blocker use was also associated with a lower positive strain (standardized β=−0.16; P=0.024) and lower (ie, less negative) early‐diastolic strain rate (standardized β=0.17; P=0.016), indicating impaired LA conduit function. Finally, beta‐blocker use was associated with a lower (less negative) late‐diastolic strain (standardized β=0.16; P=0.028) and strain rate (standardized β=0.16; P=0.041), and lower active LAEF (standardized β=−0.26; P=0.001), indicating impaired booster pump function. Angiotensin‐converting enzyme inhibitor, angiotensin receptor blocker, calcium‐channel blocker, thiazide, and spironolactone use were not associated with LA function (not shown).
With further multivariable adjustment for LV mass, LA volume, and LV EF (Model 3, Table 3), the association of beta‐blockers with impaired LA reservoir and booster pump function persisted. There was also a significant association between beta‐blocker use and a lower positive longitudinal strain (standardized β=−0.19; P=0.012) and early‐diastolic strain rate (standardized β=−0.15; P=0.039), which are measures of LA conduit function.
Sensitivity Analyses
Given the significant differences in the prevalence of coronary artery disease between subjects who were taking beta‐blockers and those who were not, we performed sensitivity analyses in which subjects with a history of coronary artery disease were excluded (Table 4). As in the overall study population, in these sensitivity analyses, beta‐blocker use was significantly associated with LA function. The numeric value of the point estimates for standardized coefficients in these analyses were, in general, similar to or greater than the estimates in the overall population.
Table 4.
Sensitivity Analyses Showing Unadjusted and Adjusted Standardized Regression Coefficients for the Association of Left Atrial Measures With Beta‐blocker Use, After Excluding Subjects With CAD (nincluded=146)
| Left Atrial Parameters | Model 1 Estimated Standardized β Coefficient (P Value) | Model 2 Estimated Standardized β Coefficient (P Value) | Model 3 Estimated Standardized β Coefficient (P Value) |
|---|---|---|---|
| Reservoir function | |||
| Total longitudinal strain | −0.22 (0.007) | −0.28 (0.002) | −0.29 (0.002) |
| LA expansion index | −0.27 (0.001) | −0.34 (<0.001) | −0.32 (<0.001) |
| Conduit function | |||
| Positive longitudinal strain | −0.14 (0.078) | −0.21 (0.02) | −0.27 (0.003) |
| Early‐diastolic strain ratea | 0.21 (0.005) | 0.26 (0.002) | 0.25 (0.004) |
| Passive LA ejection fraction | −0.08 (0.28) | −0.10 (0.205) | −0.09 (0.28) |
| Booster pump function | |||
| Negative longitudinal straina | 0.21 (0.010) | 0.23 (0.01) | 0.19 (0.036) |
| Late‐diastolic strain ratea | 0.23 (0.006) | 0.24 (0.008) | 0.26 (0.004) |
| Active LA emptying fraction | −0.23 (0.001) | −0.29 (0.002) | −0.28 (<0.001) |
Model 1: adjusted for age, sex, and race. Model 2: adjusted for variables in Model 1 plus body mass index, systolic and diastolic blood pressure, presence of diabetes mellitus, use of angiotensin‐converting enzyme inhibitors, angiotensin receptor blockers, calcium‐channel blockers, spironolactone, and diuretics. Model 3: adjusted for variables in Model 2 plus left atrial volume, left ventricular mass, and left ventricular ejection fraction. LA indicates left atrium.
Positive coefficients in diastolic strain and strain rate indicate “less negative values” and therefore worse left atrial function.
Given the significant differences in pulse pressure amplification between subjects who were receiving beta‐blockers and those who were not, we performed sensitivity analyses regarding the association of beta‐blocker use with LA function among subjects with available carotid tonometry data (Table 5). In this table Models 2 and 3 (multivariable models) include adjustment for pulse pressure amplification. It can be observed that in models that adjusted for various confounders (including measures of LV structure and function and central‐to‐brachial pulse pressure amplification, Model 3), beta‐blocker use remained predictive of LA function, suggesting that the observed relationship between beta‐blocker use and parameters of LA function is, at least in part, independent of the effect of beta‐blockers on central pressures.
Table 5.
Sensitivity Analyses Showing Unadjusted and Adjusted Standardized Regression Coefficients for the Association of Measures of Left Atrial Measures With Beta‐blocker Use, Among Subjects With Available Carotid Tonometry Data (n Included=176)
| Left Atrial Parameters | Model 1 Estimated Standardized β Coefficient (P Value) | Model 2 Estimated Standardized β Coefficient (P Value) | Model 3 Estimated Standardized β Coefficient (P Value) |
|---|---|---|---|
| Reservoir function | |||
| Total longitudinal strain | −0.15 (0.052) | −0.19 (0.022) | −0.20 (0.017) |
| LA expansion index | −0.23 (0.003) | −0.25 (0.003) | −0.24 (0.004) |
| Conduit function | |||
| Positive longitudinal strain | −0.12 (0.092) | −0.15 (0.062) | −0.18 (0.028) |
| Early‐diastolic strain ratea | 0.18 (0.012) | 0.20 (0.01) | 0.19 (0.02) |
| Passive LA ejection fraction | −0.12 (0.09) | −0.10 (0.18) | −0.07 (0.36) |
| Booster pump function | |||
| Negative longitudinal straina | 0.11 (0.162) | 0.15 (0.073) | 0.19 (0.036) |
| Late‐diastolic strain ratea | 0.13 (0.10) | 0.17 (0.055) | 0.19 (0.029) |
| Active LA emptying fraction | −0.17 (0.03) | −0.20 (0.022) | −0.15 (0.093) |
Model 1: adjusted for age, sex, and race. Model 2: adjusted for variables in Model 1 plus central‐to‐brachial pulse pressure amplification, body mass index, systolic and diastolic blood pressure, presence of diabetes mellitus, coronary artery disease, use of angiotensin‐converting enzyme inhibitors, angiotensin receptor blockers, calcium‐channel blockers, spironolactone, and diuretics. Model 3: adjusted for variables in Model 2 plus LA volume, left ventricular mass, and left ventricular ejection fraction. LA indicates left atrium.
Positive coefficients in diastolic strain and strain rate indicate “less negative values” and therefore worse LA function.
Discussion
We demonstrate for the first time that among hypertensive subjects without heart failure and AF, beta‐blocker use is significantly associated with impaired LA reservoir, conduit, and booster pump function. This association may underlie the increased risk of atrial arrhythmia and stroke that has been observed with the use of these agents in patients with hypertension.11
Left Atrial Remodeling in Hypertension
Structural and functional remodeling of the LA is seen early in the course of hypertension. In a cross‐sectional study using 2‐dimensional echocardiographic volumes and spectral Doppler, Eshoo et al4 found that reservoir and conduit LA functions were decreased, whereas booster function was increased, among participants with untreated mild hypertension compared to healthy volunteers. In that study LA volumes were increased in association with the degree of LVH, indicating the presence of structural remodeling. As hypertensive heart disease progresses, booster and booster reserve function (an index of the degree of booster function increase with exercise) also decrease.5, 6 Structural and functional remodeling of LA have been associated with an increased risk of cardiovascular morbidity and mortality in population‐based studies.1, 3 In the Dallas Heart Study, LAEF (an indicator of LA reservoir function) predicted mortality even after adjustment for LA volume index, suggesting the prognostic importance of functional remodeling independent of LA structure.15 In a cohort study of participants with chronic hypertension, Kaminski et al showed that a decrease in active LAEF (an indicator of LA booster pump function) was independently associated with all‐cause mortality and major adverse cardiac events.7
Beta‐blockers, Hypertension, and Left Atrial Parameters
To the best of our knowledge, no prior study has examined the relationship between the use of specific antihypertensive agents and measures of LA mechanical function. We used cardiac MRI‐based LA volume measurements, along with feature tracking–based assessment of phasic atrial wall longitudinal deformation.14 We utilized these modalities to measure LA volumes, LA strain, and strain rate during various phases. We found that, in contrast to other antihypertensive agents (such as calcium‐channel blockers, angiotensin‐converting enzyme [ACE] inhibitors, and ARBs), beta‐blocker use was consistently associated with impaired reservoir, conduit, and booster pump LA function.
Beta‐blocker use has been associated with an increased risk of cardiovascular morbidity and mortality.16, 17 Furthermore, an atenolol‐based strategy, when compared to a losartan‐based strategy, was associated with a higher risk of AF and stroke10, 11 in the Losartan Intervention for Endpoint Reduction in Hypertension (LIFE) trial. In this randomized trial participants in the atenolol‐based group also showed less regression of LA and LV structural remodeling.8, 18 Although we found depressed LA phasic function (a marker of LA functional remodeling) in participants using beta‐blockers, there was no difference in LV mass and LA volumes (a marker of structural remodeling). This might be because, unlike LIFE trial participants, nearly two‐thirds of the participants who used beta‐blockers in our study were also receiving an ACE inhibitor or ARB. Interestingly, in a cohort study by Kaminski et al, a significantly higher proportion of participants with depressed active LAEF were taking beta‐blockers compared to those with higher LAEF (71% vs 57%, P=0.04). However, this association was not explored further in this study. In our comprehensive evaluation of LA function with use of volumetric and strain‐based parameters, we found that beta‐blocker use was associated with depressed LA function in all 3 domains (reservoir, conduit, and booster), and this association was consistently demonstrated with various measures of LA function in each domain.
Potential mechanisms linking beta‐blocker use with atrial dysfunction may include any of the following factors, alone or in combination: (1) direct negative inotropic effects of beta‐blockers on the LA myocardium19; (2) LA dysfunction secondary to negative inotropic or lusitropic effects of beta‐blockers on the LV20, 21; and (3) worsening LA‐LV‐aortic coupling due to the effects of beta‐blockers on central hemodynamics. Although we observed a slightly decreased LVEF in participants using beta‐blockers (60±8% vs 62±8%, P<0.035), the association between LA function and beta‐blocker use persisted after adjustment for LVEF. Previous studies indicate that central pulsatile hemodynamics is associated with LA remodeling and dysfunction in hypertension.22, 23 In the CAFE study,9 a prospective substudy of ASCOT, the use of atenolol‐based regimens was associated with higher central systolic and pulse pressure despite similar reduction in peripheral blood pressure when compared to amlodipine‐based regimens. In the LIFE trial,24 higher pulse pressure was observed in the atenolol‐based group when compared to the losartan‐based group, and this independently predicted increased risk of cardiovascular morbidity and mortality in these participants. We found that hypertensive subjects who were receiving beta‐blockers demonstrated a lower pulse pressure amplification, indicating greater central pulse pressure for any given level of brachial pulse pressure. Interestingly, in our sensitivity analyses examining the relationship between beta‐blocker use and LA function among subjects with available central pressure data (Table 4), beta‐blocker use remained predictive of measures of LA function after adjustment for various confounders, including carotid‐to‐brachial pulse pressure amplification. This indicates that the effects of beta‐blockers on LA function are, at least in part, independent on their effects on central pulse pressure.
Strengths and Limitations
To the best of our knowledge, this is the first study to evaluate the relationship between the use of different antihypertensive agents and phasic LA function. An additional strength of our study is the use of cardiac MRI, which provides superior quantitative data about volumes and chamber function. Furthermore, we used novel tissue‐tracking algorithms to assess tissue strain (deformation), a more direct measure of myocardial function. The high consistency of our results with various functional LA indices, and with multiple adjustments for potential confounders, adds confidence to our results. However, these results should be interpreted in the context of the study limitations. Although the observed relationships with beta‐blocker use were highly consistent and statistically significant, the magnitude of the association in multivariable analyses was modest to moderate. However, this may be due to the multiple potential factors (other than beta‐blocker use) that can have an impact on LA function. Residual confounding cannot be excluded, and our study does not firmly establish a cause‐effect relationship because of its cross‐sectional nature. Many patients were receiving combination therapy with various agents and various doses; it is possible that concomitant therapy may have confounded the association between beta‐blocker use and LA function. However, this is unlikely given that there was no evidence of an association between other classes of antihypertensives and LA function parameters in the sample. In addition, we adjusted for concomitant drug class. More detailed modeling of the potential confounding effects of specific drugs in each class and at different dosages was not possible due to the moderate sample size. Another limitation is that we did not account for the duration of hypertension or of drug therapy with various agents. Interestingly, because beta‐blockers were historically a first‐line medication, beta‐blocker use could reflect a longer duration of hypertension, which in turn would reflect greater negative effects of hypertension on LA function. However, we note that adjustment for other structural factors (including LV mass, end‐diastolic volume, and EF) did not attenuate the observed associations. Furthermore, we utilized a convenience sample to recruit our study participants at our VA Medical Center, which limits the generalizability of the findings. Our study population was therefore composed predominantly of males. Although we included sex as a covariate in multivariable regression models, further validation of our observations in samples that include a larger number of women should be performed. Finally, there was a significant difference in the prevalence of coronary artery disease between the 2 groups. Although we performed sensitivity analyses to address these differences, residual confounding cannot be fully excluded.
Conclusions
In summary, in this cross‐sectional analysis of participants with chronic hypertension, we found a significant association of beta‐blocker use with impaired reservoir, conduit, and booster pump LA function when compared to other antihypertensive agents. This association could underlie the increased risk of AF and stroke seen with the use of beta‐blockers (as opposed to other antihypertensive agents) in recent trials.
Sources of Funding
This study was supported by NIH grants R56HL‐124073‐01A1 (Chirinos), R01 HL 121510‐01A1 (Chirinos), 5‐R21‐AG‐043802‐02 (Chirinos), and a VISN‐4 research grant from the Department of Veterans Affairs (Chirinos).
Disclosures
Chirinos has received consulting honoraria from Bristol‐Myers Squibb, OPKO Healthcare, Fukuda Denshi, Microsoft, Vital Labs, and Merck. He received research grants from the National Institutes of Health, American College of Radiology Network, Fukuda Denshi, Bristol‐Myers Squibb, Microsoft, and CVRx Inc and device loans from AtCor Medical. Chirinos is named as inventor in a University of Pennsylvania patent application for the use of inorganic nitrates/nitrites for the treatment of HF with preserved EF. Other authors have no disclosures.
Supporting information
Video S1. Representative video of atrial tissue tracking using cine SSFP‐MRI images.
(J Am Heart Assoc. 2017;6:e005163. DOI: 10.1161/JAHA.116.005163.)
References
- 1. Benjamin EJ, Dagostino RB, Belanger AJ, Wolf PA, Levy D. Left atrial size and the risk of stroke and death—the Framingham Heart‐Study. Circulation. 1995;92:835–841. [DOI] [PubMed] [Google Scholar]
- 2. Tsioufis C, Stougiannos P, Taxiarchou E, Skiadas I, Chatzis D, Thomopoulos C, Lalos S, Stefanadis C, Kallikazaros I. The interplay between haemodynamic load, brain natriuretic peptide and left atrial size in the early stages of essential hypertension. J Hypertens. 2006;24:965–972. [DOI] [PubMed] [Google Scholar]
- 3. Hoit BD. Left atrial size and function: role in prognosis. J Am Coll Cardiol. 2014;63:493–505. [DOI] [PubMed] [Google Scholar]
- 4. Eshoo S, Ross DL, Thomas L. Impact of mild hypertension on left atrial size and function. Circ Cardiovasc Imaging. 2009;2:93–99. [DOI] [PubMed] [Google Scholar]
- 5. Tan YT, Wenzelburger F, Lee E, Nightingale P, Heatlie G, Leyva F, Sanderson JE. Reduced left atrial function on exercise in patients with heart failure and normal ejection fraction. Heart. 2010;96:1017–1023. [DOI] [PubMed] [Google Scholar]
- 6. Soullier C, Niamkey JT, Ricci JE, Messner‐Pellenc P, Brunet X, Schuster I. Hypertensive patients with left ventricular hypertrophy have global left atrial dysfunction and impaired atrio‐ventricular coupling. J Hypertens. 2016;34:1615–1620. [DOI] [PubMed] [Google Scholar]
- 7. Kaminski M, Steel K, Jerosch‐Herold M, Khin M, Tsang S, Hauser T, Kwong RY. Strong cardiovascular prognostic implication of quantitative left atrial contractile function assessed by cardiac magnetic resonance imaging in patients with chronic hypertension. J Cardiovasc Magn Reson. 2011;13:42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Devereux RB, Dahlof B, Gerdts E, Boman K, Nieminen MS, Papademetriou V, Rokkedal J, Harris KE, Edelman JM, Wachtell K. Regression of hypertensive left ventricular hypertrophy by losartan compared with atenolol: the Losartan Intervention for Endpoint Reduction in Hypertension (LIFE) trial. Circulation. 2004;110:1456–1462. [DOI] [PubMed] [Google Scholar]
- 9. Williams B, Lacy PS, Thom SM, Cruickshank K, Stanton A, Collier D, Hughes AD, Thurston H, O'Rourke M; CAFE Investigators; Anglo‐Scandinavian Cardiac Outcomes Trial Investigators; CAFÉ Steering Committee and Writing Committee . Differential impact of blood pressure‐lowering drugs on central aortic pressure and clinical outcomes: principal results of the Conduit Artery Function Evaluation (CAFE) study. Circulation. 2006;113:1213–1225. [DOI] [PubMed] [Google Scholar]
- 10. Kjeldsen SE, Dahlof B, Devereux RB, Julius S, Aurup P, Edelman J, Beevers G, de Faire U, Fyhrquist F, Ibsen H, Kristianson K, Lederballe‐Pedersen O, Lindholm LH, Nieminen MS, Omvik P, Oparil S, Snapinn S, Wedel H; Group LS . Effects of losartan on cardiovascular morbidity and mortality in patients with isolated systolic hypertension and left ventricular hypertrophy: a Losartan Intervention for Endpoint Reduction (LIFE) substudy. JAMA. 2002;288:1491–1498. [DOI] [PubMed] [Google Scholar]
- 11. Wachtell K, Lehto M, Gerdts E, Olsen MH, Hornestam B, Dahlof B, Ibsen H, Julius S, Kjeldsen SE, Lindholm LH, Nieminen MS, Devereux RB. Angiotensin II receptor blockade reduces new‐onset atrial fibrillation and subsequent stroke compared to atenolol: the Losartan Intervention for End Point Reduction in Hypertension (LIFE) study. J Am Coll Cardiol. 2005;45:712–719. [DOI] [PubMed] [Google Scholar]
- 12. Chirinos JA, Segers P, De Buyzere ML, Kronmal RA, Raja MW, De Bacquer D, Claessens T, Gillebert TC, St John‐Sutton M, Rietzschel ER. Left ventricular mass: allometric scaling, normative values, effect of obesity, and prognostic performance. Hypertension. 2010;56:91–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Imai M, Ambale Venkatesh B, Samiei S, Donekal S, Habibi M, Armstrong AC, Heckbert SR, Wu CO, Bluemke DA, Lima JA. Multi‐Ethnic Study of Atherosclerosis: association between left atrial function using tissue tracking from cine MR imaging and myocardial fibrosis. Radiology. 2014;273:703–713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Evin M, Redheuil A, Soulat G, Perdrix L, Ashrafpoor G, Giron A, Lamy J, Defrance C, Roux C, Hatem SN, Diebold B, Mousseaux E, Kachenoura N. Left atrial aging: a cardiac magnetic resonance feature‐tracking study. Am J Physiol Heart Circ Physiol. 2016;310:H542–H549. [DOI] [PubMed] [Google Scholar]
- 15. Gupta S, Matulevicius SA, Ayers CR, Berry JD, Patel PC, Markham DW, Levine BD, Chin KM, de Lemos JA, Peshock RM, Drazner MH. Left atrial structure and function and clinical outcomes in the general population. Eur Heart J. 2013;34:278–285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Wiysonge CS, Bradley HA, Volmink J, Mayosi BM, Mbewu A, Opie LH. Beta‐blockers for hypertension. Cochrane Database Syst Rev. 2012;11:CD002003. [DOI] [PubMed] [Google Scholar]
- 17. Bangalore S, Sawhney S, Messerli FH. Relation of beta‐blocker‐induced heart rate lowering and cardioprotection in hypertension. J Am Coll Cardiol. 2008;52:1482–1489. [DOI] [PubMed] [Google Scholar]
- 18. Gerdts E, Wachtell K, Omvik P, Otterstad JE, Oikarinen L, Boman K, Dahlof B, Devereux RB. Left atrial size and risk of major cardiovascular events during antihypertensive treatment: Losartan Intervention for Endpoint Reduction in Hypertension trial. Hypertension. 2007;49:311–316. [DOI] [PubMed] [Google Scholar]
- 19. Varma DR, Shen H, Deng XF, Peri KG, Chemtob S, Mulay S. Inverse agonist activities of β‐adrenoceptor antagonists in rat myocardium. Br J Pharmacol. 1999;127:895–902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Brodde OE, Michel MC. Adrenergic and muscarinic receptors in the human heart. Pharmacol Rev. 1999;51:651–690. [PubMed] [Google Scholar]
- 21. Triposkiadis F, Karayannis G, Giamouzis G, Skoularigis J, Louridas G, Butler J. The sympathetic nervous system in heart failure physiology, pathophysiology, and clinical implications. J Am Coll Cardiol. 2009;54:1747–1762. [DOI] [PubMed] [Google Scholar]
- 22. Miyoshi H, Mizuguchi Y, Oishi Y, Iuchi A, Nagase N, Ara N, Oki T. Early detection of abnormal left atrial‐left ventricular‐arterial coupling in preclinical patients with cardiovascular risk factors: evaluation by two‐dimensional speckle‐tracking echocardiography. Eur J Echocardiogr. 2011;12:431–439. [DOI] [PubMed] [Google Scholar]
- 23. Jaroch J, Rzyczkowska B, Bociaga Z, Loboz‐Rudnicka M, Kruszynska E, Rychard W, Dudek K, Poreba R, Loboz‐Grudzien K. Arterial‐atrial coupling in untreated hypertension. Blood Press. 2015;24:72–78. [DOI] [PubMed] [Google Scholar]
- 24. Fyhrquist F, Dahlof B, Devereux RB, Kjeldsen SE, Julius S, Beevers G, de Faire U, Ibsen H, Kristianson K, Lederballe‐Pedersen O, Lindholm LH, Nieminen MS, Omvik P, Oparil S, Hille DA, Lyle PA, Edelman JM, Snapinn SM, Wedel H; LIFE Study Group . Pulse pressure and effects of losartan or atenolol in patients with hypertension and left ventricular hypertrophy. Hypertension. 2005;45:580–585. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Video S1. Representative video of atrial tissue tracking using cine SSFP‐MRI images.


