Figure 2. Methyl-TROSY spectra of H2AK15ub-H2B and NCPH2AK15ub.
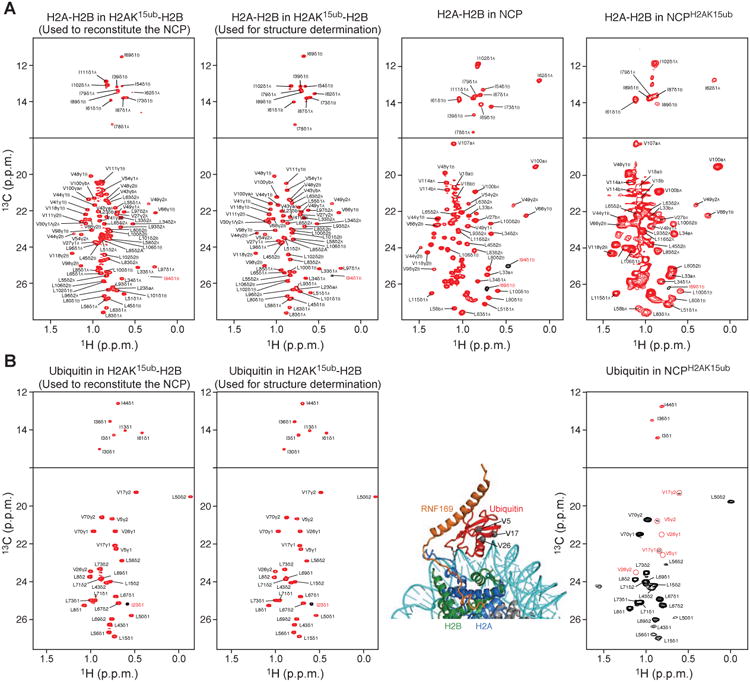
(A) Methyl-TROSY spectra of Ile, Val, Leu methyl-labeled H2A-H2B in the context of H2AK15ub-H2B, NCP and NCPH2AK15ub. From left to right: First panel corresponds to the enzymatically ubiquitylated long version of H2A-H2B used to reconstitute the NCP. Second panel corresponds to the enzymatically ubiquitylated short version of H2A-H2B used for NMR structure determination of H2AK15ub-H2B–RNF169. Third panel corresponds to the NCP reconstituted with the non-ubiquitylated long version of H2A-H2B. Fourth panel corresponds to ubiquitylated NCP reconstituted with the long version of H2AK15ub-H2B in the first panel.
(B) Methyl-TROSY spectra of Ile, Val, Leu methyl-labeled ubiquitin in the context of H2AK15ub-H2B and NCPH2AK15ub described in A. In the right spectrum, the ubiquitin methyl signals that extensively broadened in the context of NCPH2AK15ub but not in H2AK15ub-H2B (left spectra) are highlighted with red circles. The corresponding residues (Val5, Val17 and Val26) are shown as gray spheres in the NCPH2AK15ub–RNF169 complex structure.
