Figure 4.
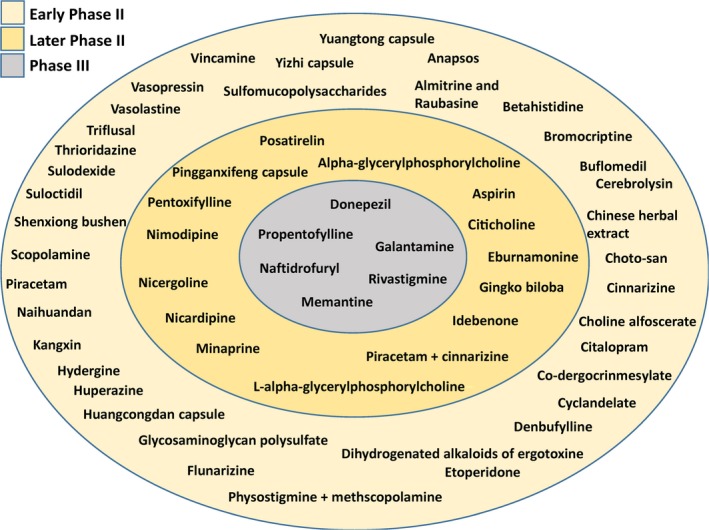
Drug development status of medications for patients with vascular cognitive impairment. Phase III was operationally defined retrospectively as randomized controlled trials with ≥300 participants, later phase II as ≥100 to 299 participants, and early phase II as <100 participants. Data from trials with untreated controls (mostly receiving a placebo) were used to generate the figure. A single large trial (>300 participants) of the calcium channel blockers nicardipine vs clentiazem was not included because there was no untreated control arm.
