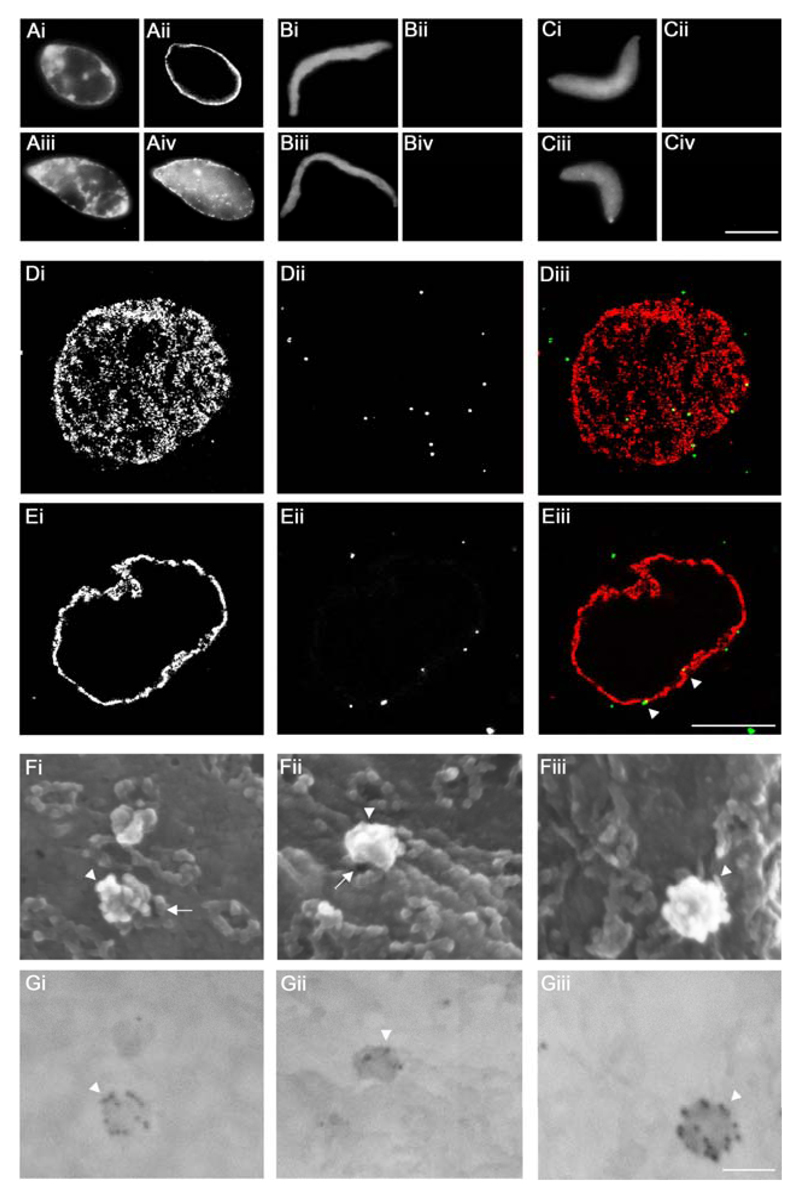
Figure 4. Targeting of HSV1 capsids to NPCs on Xenopus nuclei in vitro.
A to C. Functional (A), Imp β45-462 (B) or BAPTA (C) nuclei were reconstituted in vitro. Completion of nuclear assembly was monitored by epi-fluorescence microscopy by DNA staining with Hoechst 33258 (i and iii), NPC staining with fluorescently labeled mAb414 (ii) and the transport capacity of the assembled NPCs was confirmed by the nuclear import of TRITC-NLS-BSA (iv). Scale bar: 10 µm. D, E. Binding of viral 0.5 M KCl capsids extracted from extracellular particles of HSV1-GFPVP26 (ii and iii, green, white arrowheads) to Xenopus nuclei was analyzed using confocal fluorescence laser scanning microscopy and a serial z-slicing of 0.37 μm optical sections. A surface view (D) and one midsection (E) of a representative nucleus are shown. NPCs were labeled with Imp β45-462-TRITC (i and iii, red). Scale bar: 10 µm. F, G. HSV1-GFPVP26 viral 0.5 M capsids were incubated with Xenopus nuclei harboring functional NPCs for 45 min, labeled with a polyclonal rabbit antiserum raised against intact HSV1 capsids (Remus, bleed V) and colloidal gold with a diameter of 12 nm coated with anti-rabbit antibodies. The specimens were analyzed by field emission scanning electron microscopy. (F) 3D surface topography of reconstituted nuclei from different views in the in-lens images (Fig. 4Fi-iii). The same areas were imaged through a backscatter electron detector revealing the positions of gold-conjugated antibodies (Fig. 4Gi-iii; inverted color mode). HSV1 capsids (white arrowheads) are bound to the cytoplasmic face of the NPCs (white arrows). Scale bar: 100 nm.