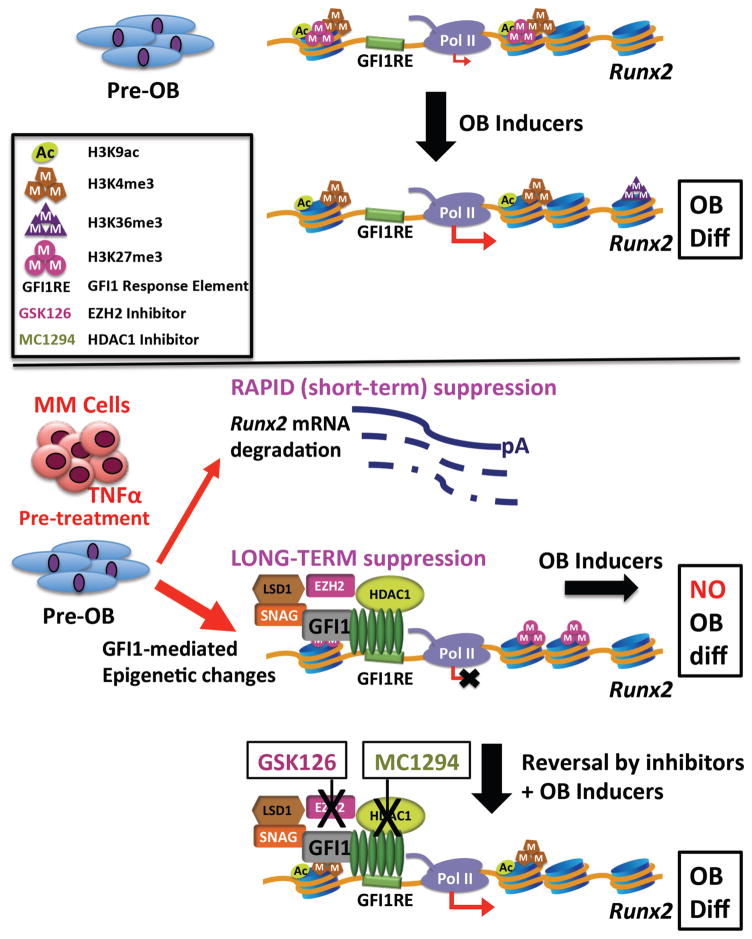
Figure 7. Schematic of the mechanism of GFI1-induced epigenetic repression of the Runx2 locus in MM exposed pre-OB.
In proliferating pre-OB cells, Runx2-P1 is in a poised bivalent configuration with paused Pol II and prominent levels of activation-ready promoter chromatin marks H3K4me3 and H3K9ac, as well as H3K27me3, with low levels of basal transcription. OB differentiation induction stimulates increased accumulation of these active chromatin marks, as well as release of Pol II into the Runx2 structural region as marked by increased Pol II Ser2P-CTD and accumulation of the H3K36me3 mark. MM exposure acts in a dual mode to repress Runx2 expression. The rapid TNFα-induced decrease in Runx2 mRNA is mediated by increased mRNA degradation. However, this is insufficient to block induction of OB differentiation. The sustained suppression of OB differentiation requires modifications of the Runx2 chromatin architecture. GFI1 binds to Runx2 and facilitates recruitment of histone co-repressors HDAC1, LSD1 and EZH2, which results in decreased active H3K9ac and H3K4me3 and increased repressive H3K27me3 chromatin marks, causing an epigenetic block refractory to transcriptional activation in response to OB differentiation signals. Inhibition of either HDAC1 or EZH2 can reverse the inhibition and allow OB differentiation.