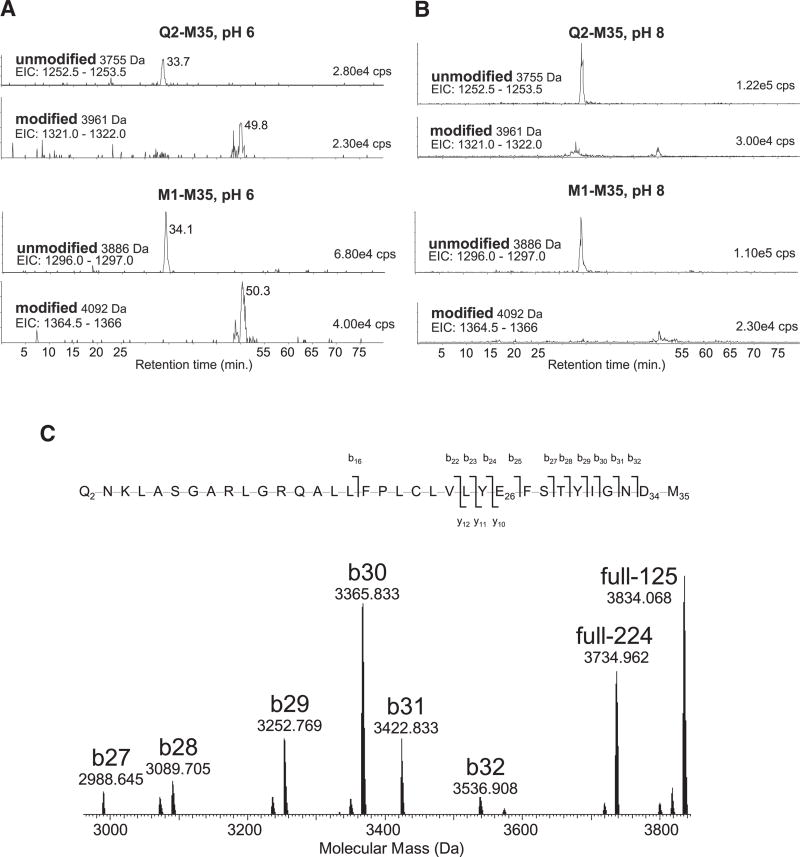
Figure 6. Mass Spectrometry of Peptide Q2-M35.
(A–C) CNBr-generated peptides were separated by reverse-phase chromatography with online electrospray-ionization mass spectrometry. The MdfA N-terminal peptides M1-M35 and Q2-M35 containing E26 and D34 have a mass of 3755 or 3886 Da, respectively. Single modification with DCCD resulted in the respective peptides of mass 3961 or 4092 Da. (A) and (B) show extracted ion chromatograms (EIC: m/z range) for the triply charged ions that predominate after ESI of these four peptide species. DCCD treatment at pH 6.0 (A) and DCCD treatment at pH 8.0 (B) are shown. The intensity of each peak is given in the top right of each panel in cps (counts per second, arbitrary units). The traces shown are scaled to the most abundant species. In (C), the 3961 Da DCCD modified peptide (A) was analyzed by high-resolution FT-ICR tandem mass spectrometry with collisionally activated dissociation (CAD). The higher mass range of the deconvoluted molecular mass spectrum is shown for the ions indicated. The identified b- and y-ions are shown mapped onto the primary sequence (b-ions, of N-terminal origin are indicated by a slash rising to the left; y-ions, of C-terminal origin, with a slash dropping to the right). Explanation: DCCD modification adds 206.178 Da, and M35 modification to homoserine lactone reduces the mass by 48.003 Da. Mass accuracy of the ions is provided in Table S1. The ions b25 and b27–b32, which carry E26 but not D34, also do not carry the DCCD modification. Unmodified product ions could arise from a modified parent ion only if neutral loss of intact DCCD (−206 Da) occurred during CAD. The ions shown at 3734.965 (full −224) and 3834.068 (full −125) correspond to neutral losses of −224 and −125 Da, respectively, from the precursor ion, in agreement with previous tandem mass spectrometry analysis of DCCD-modified peptides (Seeger et al., 2009). Thus the only explanation for the intense b27–b32 unmodified product ions is that the DCCD modification is located predominantly on D34. See also Table S1.