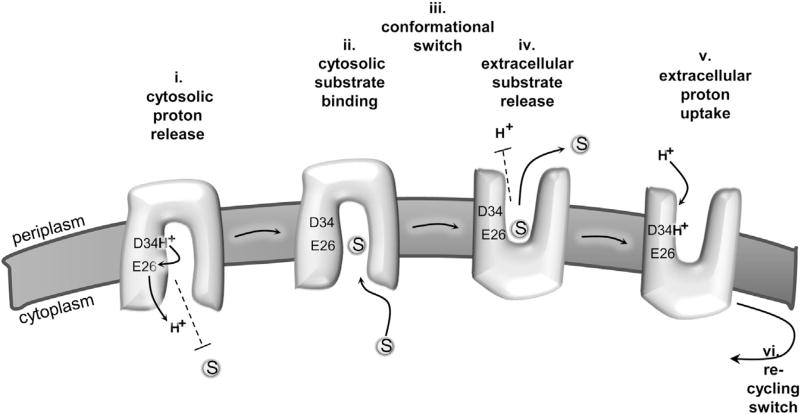
Figure 7. Model for MdfA-Catalyzed Substrate/H+ Antiport.
(i) Proton is released from D34 to the cytosol in an E26-mediated manner. Substrates are prevented from binding to MdfA in the proton bound state (dashed line).
(ii) Upon spontaneous deprotonation, substrates bind from the cytosol, to a site that is distinct from D34.
(iii) Substrate binding triggers a conformational switch that exposes the multidrug-binding pocket to the extracellular side (periplasm). (iv) Protons cannot bind to MdfA (dashed line) until substrate is released to the extracellular side. (v) A proton binds to D34 from the extracellular side.
(vi) The proton-binding event triggers a conformational switch that recycles MdfA to the inward-facing conformation.