Fig. 8.
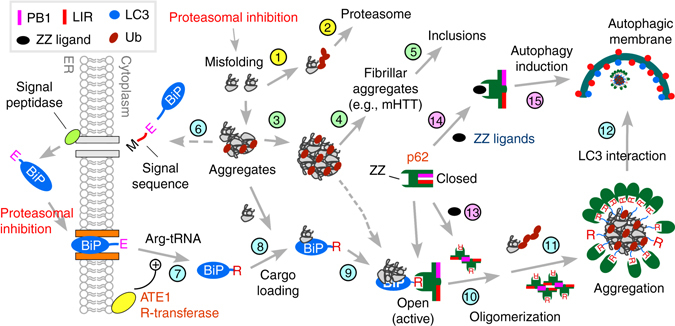
A model illustrating the N-end rule pathway in the modulation of autophagy. Cytosolic misfolded proteins are normally degraded through ubiquitination and proteasomal degradation (Steps 1 and 2). Some misfolded proteins (e.g., mHTT) may be prone to aggregation (Step 3) and, thus, are difficult to be degraded by the proteasome. In neurodegenerative diseases, these proteasome-resistant oligomeric aggregates grow into fibrillar forms and large inclusions (Steps 4 and 5). In protein quality control, cells sense the accumulation of such misfolded proteins and their aggregates, inducing Nt-arginylation of ER-residing proteins, such as BiP (Steps 6 and 7). Nt-arginylated ER proteins accumulate in the cytosol. Cytosolic R-BiP is associated with misfolded protein cargoes (Step 8) and binds the ZZ domain of p62 through its Nt-Arg (Step 9). Upon binding to Nt-Arg, p62 undergoes a conformational change, exposing PB1 and LC3-interaction domain (Step 10). This accelerates self-polymerization of p62, resulting in the formation of cargo–R-BiP–p62 protein aggregates (Step 11). The binding with Nt-Arg also enhances p62 interaction with LC3 on autophagic membranes (Step 11), leading to the delivery of cargo–R-BiP–p62 complexes into autophagosomes (Step 12). In this study, we developed small molecule ligands to p62 ZZ domain. Our results suggest that ligand-bound p62 acts as an autophagic inducer (Steps 14 and 15) by enhancing the synthesis of LC3-I and its conversion into LC3-II and promoting the formation of autophagosomes
