Fig. 3.
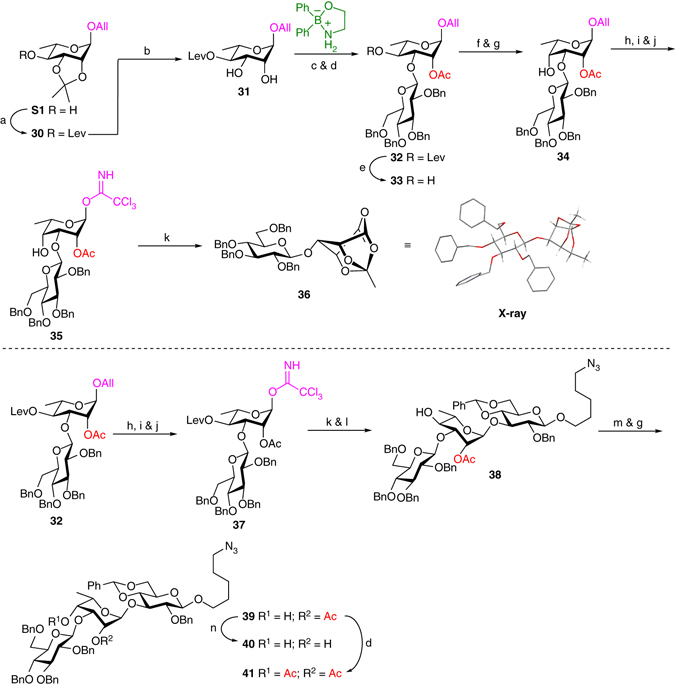
Second-generation synthesis of protected trisaccharides. Reagents and conditions: a Lev2O, py, DMAP, 50 °C, 2 h, 99%; b 80% aq. HOAc, 60 °C, 6 h, 82%; c chloride donor S33, 2-aminoethyl diphenylborinate (0.25 equivalent), Ag2O, CH3CN, 4 Å MS, 60 °C, overnight, 74%; d Ac2O, py, DMAP, RT, 3–4 h, 98% (for 32); 94% (for 41); e H2NNH2.HOAc, DCM, MeOH, RT, overnight 82%; f Dess–Martin periodinane, DCE, 70 °C, 1 h; g NaBH4, MeOH/DCM 5:1, −10 °C to RT, 71% (for 34, over two steps); 85% (for 39, over two steps); h [Ir(COD){PMe(C6H5)2}2]+.PF6 −, H2, THF, RT, 1 h; i I2, THF, H2O, RT, 2 h; j CCl3CN, Cs2CO3, DCM, Me2CO, RT, 2 h, 65% (for 35, over three steps); 81% (for 37, over three steps); k acceptor 13, TMSOTf, 4 Å MS (only for 38), Et2O/DCE 5:1, −10 °C, 10 min, 41% (for 36); l H2NNH2.H2O, py, HOAc, 0 °C to RT, overnight, 77% (over two steps); m PDCP, DMSO, Et3N, DCM, −10 °C to RT, 1 h; n NaOMe, MeOH/DCM 2:1, RT, overnight, 81%. Ac 2 O acetic anhydride, CCl 3 CN trichloroacetonitrile, COD cyclooctadienyl, DMAP 4-(dimethylamino)pyridine, DMSO dimethylsulfoxide, Et 3 N triethylamine, HOAc acetic acid, Lev 2 O levulinic anhydride, PDCP phenyl dichlorophosphate, py pyridine, RT room temperature, THF tetrahydrofuran
