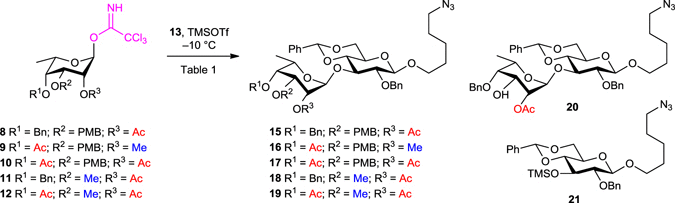
Table 1.
Synthesis of protected disaccharides
| ||||||
|---|---|---|---|---|---|---|
| Entry | Donor (equivalents) | Solventa | 4 Å MSb/time (h) | TMSOTf (equivalents) | Product yield (%)c | Ratio α/β d |
| 1 | 8 (1.3) | DCE | +/21 | 0.2 | 15 (30)e | α only |
| 2 | 8 (2.0) | Et2O | +/1 | 0.2 | 15 (43)f | α only |
| 3 | 8 (1.5) | Et2O | +/8 | 0.2 | 15 (78) | α only |
| 4 | 8 (2.0) | Et2O | −/0.2 | 0.02 | 15 (95) | α only |
| 5 | 9 (2.0) | DCE | +/0.2 | 0.2 | 16 (51) | α only |
| 6 | 9 (2.0) | Et2O | −/0.2 | 0.01 | 16 (90) | α only |
| 7 | 10 (2.0) | DCE | +/0.2 | 0.2 | 17 (44) | α only |
| 8 | 10 (2.0) | Et2O/DCE 5:1g | −/0.2 | 0.01 | 17 (58) | α only |
| 9 | 11 (2.0) | Et2O | −/0.2 | 0.01 | 18 (81) | α only |
| 10 | 12 (2.0) | Et2O | −/0.2 | 0.01 | 19 (76) | α only |
DCE 1,2-dichloroethane, Et 2 O diethyl ether, MS molecular sieves, TMSOTf trimethylsilyltrifluoromethanesulfonate
aAnhydrous solvent over molecular sieves (~0.05 M)
bWith (+) or without (−) freshly activated powdered molecular sieves
cIsolated yield
dDetermined by 1H NMR
eDisaccharide 20 was isolated as the major compound
fSilylated derivative 21 was isolated in 42% yield
gDCE was added to ensure the solubility of donor
