Table 2.
Synthesis of protected trisaccharides
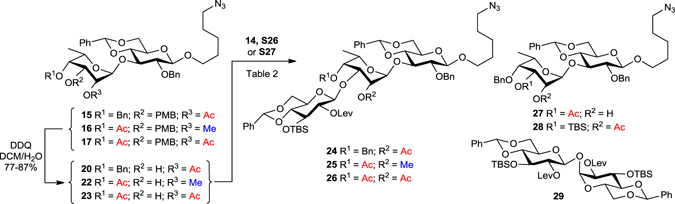
| |||||||
|---|---|---|---|---|---|---|---|
| Entry | Acceptor | Donora | Promoterb | Solventc | T (°C) (time (h)) | Product | Yield (%)d |
| 1 | 22 | 14 | NIS, AgOTf | Et2O/DCE | −10 (0.2) | 25 | 65e |
| 2 | 23 | 14 | NIS, AgOTf | Et2O/DCE | −10 (0.2) | 26 | 50e |
| 3 | 20 | 14 | NIS, AgOTf | Et2O/DCE | −10 (0.2) | 24 | NDf |
| 4 | 20 | 14 | NIS, AgOTf | DCE | −10 (1) | 24 | NDf |
| 5 | 20 | 14 | NIS, AgOTf | DCM | −78 (3) | 24 | NDg |
| 6 | 20 | 14 | CuBr2, TBAB | DCM/DMF | 22 (72) | 27 | 90 |
| 7 | 20 | 14 | DMTST, DTBMP | DCE | 40 (48) | 24 | NDh |
| 8 | 20 | S26 (F) | SnCl2, AgOTf | Et2O/DCM | −10 (0.3) | 24 | NDf |
| 9 | 20 | S27 (PTFA) | TMSOTf | DCE | −10 (0.2) | 24 | NDf |
| 10i | 20 | S27 (PTFA) | TBSOTf | tol | 75 (2) | 28 | 60 |
AgOTf silver(I) trifluoromethanesulfonate, DCM dichloromethane, DDQ 2,3-dichloro-5,6-dicyano-1,4-benzoquinone, DMF N,N-dimethylformamide, DMTST dimethyl(methylthio)sulfonium trifluoromethanesulfonate, DTBMP 2,6-di-tert-butyl-4-methylpyridine, NIS N-iodosuccinimide, TBAB tetrabutylammonium bromide, TBSOTf tert-butyldimethylsilyl trifluoromethanesulfonate, tol toluene
aDonor was used in excess (1.5 equivalents)
bThe reaction was performed adding freshly activated powdered molecular sieves
cAnhydrous solvent over molecular sieves (~0.05 M)
dIsolated yield
eOnly the β-anomer was detected by 1H NMR
fDegradation of donor
gThe dimer 29 was detected as the major compound
hNo reaction
iInverse procedure
