Figure 1.
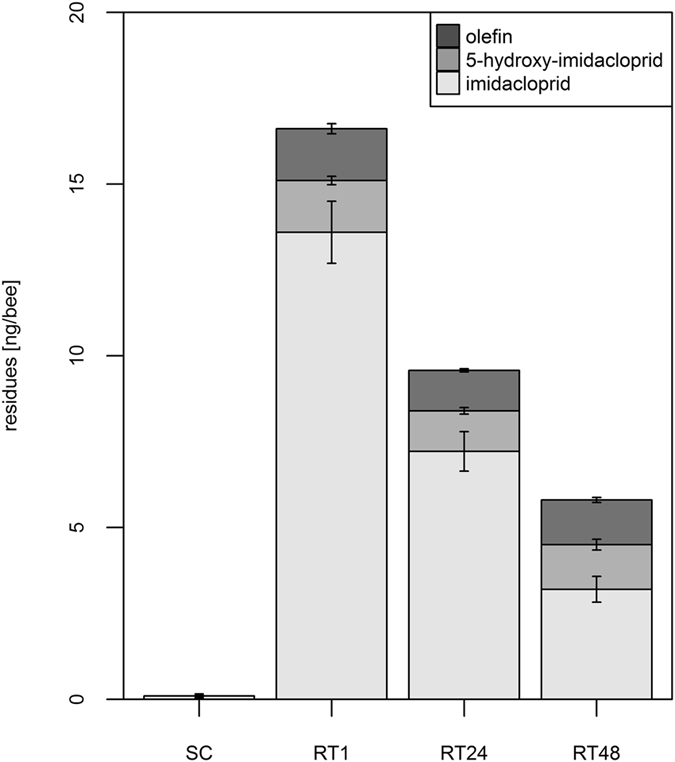
Whole-body imidacloprid residues decrease in honeybee samples over time. Individual honeybees were fed with a sugar syrup control (SC), containing only the solvent acetone but no imidacloprid or a dosage of 41 ng/bee imidacloprid (n = 150 bees/group). The honeybees were frozen after one hour (SC and RT1), 24 hours (RT24), or 48 hours (RT48) at room temperature. LC/MS/MS was used to quantify residue levels of imidacloprid, 5-hydroxy-imidacloprid, and imidacloprid-olefin. Error bars indicate standard error. The imidacloprid residue level was only significantly influenced (p < 0.001) by the time passed after ingestion. The residue level of 5-hydroxy-imidacloprid was influenced by the hive the bees originated from, the time passed after ingestion and interaction effects between both (all p < 0.001). The residue level of imidacloprid-olefin was only influenced by the interaction effects between the hive the bees originated and the time passed after ingestion (p < 0.001, see statistical report in supplement).
