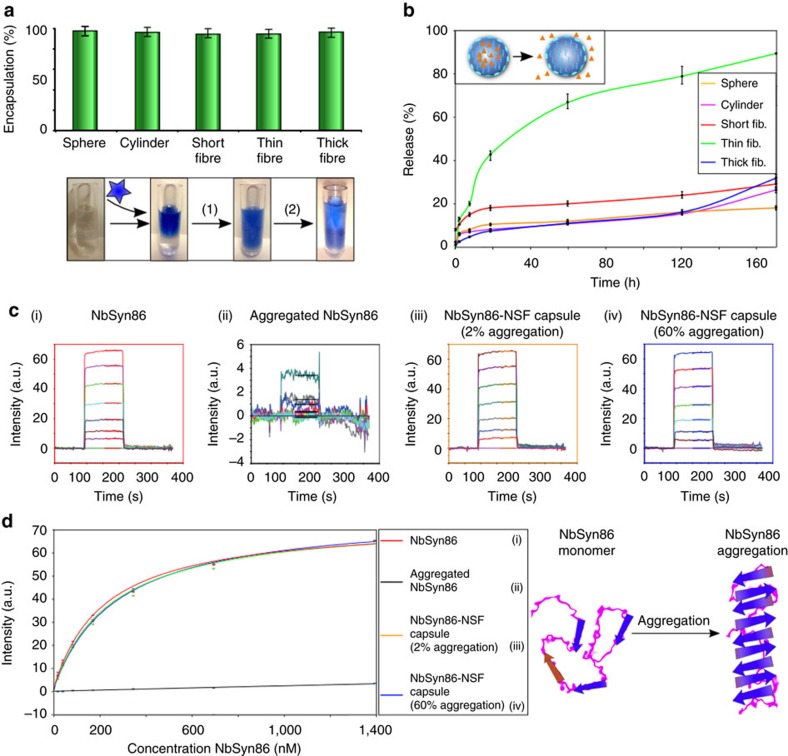
Figure 5. Antibodies stabilization by encapsulation.
(a) Encapsulation efficiency studies for the C4scFv single chain Fv domain in spheres, cylinders, short, thin and thick fibres. The indicated error bars are the s.d. of the average of three different repeats, each one measure 10 times. Insert: schematic representation of the encapsulation and release of antibiody domains by NSF micrococoons: step (1) encapsulation, step (2) release. (b) Release kinetics for C4scFv from different NSF micrococoon shapes. The indicated error bars are the s.d. of the average of three different repeats, each one measure 10 times. (c) Biacore sensorgrams of the binding of NbSyn86 to immobilized α-synuclein: (i) a control sample of monomeric NbSyn86, (ii) NbSyn86 after encapsulation and release treatment in the absence of NSF, (iii) NbSyn86 released from gelled NSF micrococoons (which contain ca. 2% of aggregated NSF), (iv) NbSyn86 released from gelled micrococoons which contain ca. 60% of aggregated NSF. (d) Graph of the equilibrium binding values for the different released NbSyn86 samples (as shown in c) versus the initial (pre-encapsulation) concentration of NbSyn86. The data were fitted to a 1:1 bimolecular binding model to estimate the affinity constant of NbSyn86 for α-synuclein, and were also used to estimate the loss of activity between samples. The indicated error bars are the s.d. of the average of three different repeats, each one measure five times.