Figure 4.
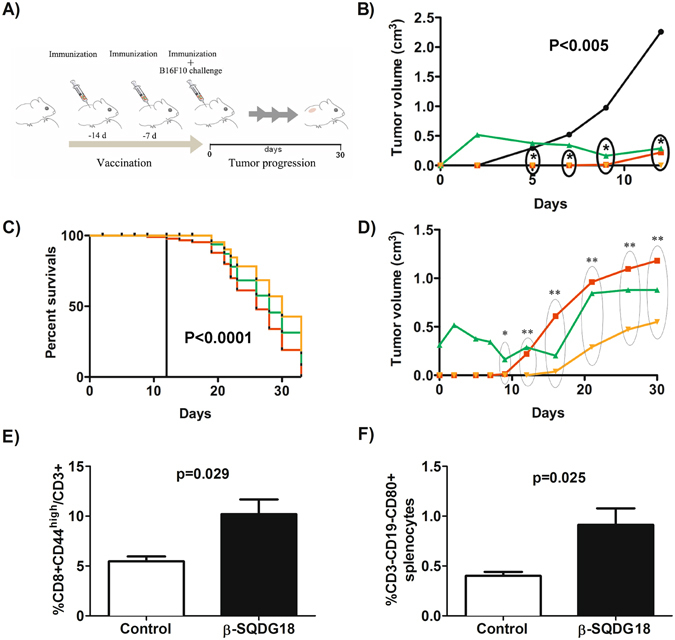
Protective effect of hgp10025–33 peptide formulated with synthetic β-SQDG18 as adjuvant in an experimental model of anti-melanoma vaccine. (A) Experimental design of immunization in C57BL/6 J mice challenged by 105 B16F10 melanoma cells (day 0). The animals were pretreated with the adjuvanted antigen hgp10025–33 peptide (100 μg mouse/injection) on days −14, −7 and 0. For the experiment with β-SQDG18 (600 μg mouse/injection), the antigen was vigorously mixed with a homogeneous suspension of the molecule prior to injection. Mice immunized with the melanoma epitope in association to either CPG oligodeoxynucleotide (30 μg/injection) or Freund’s adjuvants (1:1 vol/vol) were used as positive controls. Unimmunized mice were used as negative control. Tumour volume of melanoma lesions and survival rate were monitored for 30 days after challenging. Each group was composed of eight animals; (B) Tumour growth and (C) percent survival in the four groups of mice. Statistically significant difference between the negative control and each of the three immunized groups of mice is indicated. Black line: negative control; Red line: antigen plus β-SQDG18; Green line: antigen plus Freund’s adjuvant; Orange line: antigen plus CpG; (D) Detail of tumour growth in response to vaccination with the three adjuvants. Groups are indicated by colours as above Asterisks indicate statistically significant differences in comparison to negative control. *P < 0.01; **P < 0.002; (E) Percentage of CD8+CD44high memory T cell in splenocytes of tumour-challenged mice in the negative control group or after vaccination by β-SQDG18 as adjuvant (black column); (F) Percentage of CD3-CD19-CD80+ cells in splenocytes of tumour challenged mice in the negative control group and after vaccination by β-SQDG18 as adjuvant (black column).
