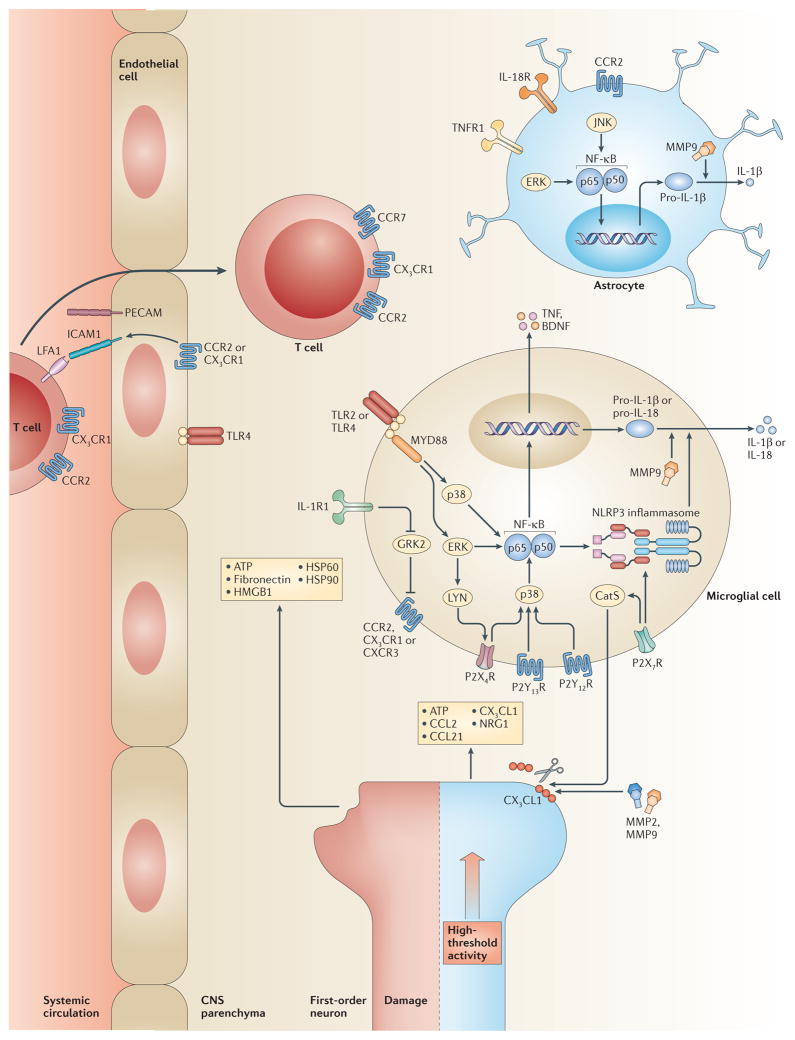
Figure 2. Initiation of central immune signalling.
Damage to or high-threshold activation of first-order neurons by noxious stimuli induces the release of ATP, CC-chemokine ligand 2 (CCL2), CCL21 and neuregulin 1 (NRG1), as well as endogenous danger signals to initiate central immune signalling in the dorsal horn of the spinal cord. CCL2 is released and rapidly upregulated by neurons following depolarization, and signals via its cognate receptor CC-chemokine receptor 2 (CCR2) on microglia. CX3C-chemokine ligand 1 (CX3CL1) is liberated from the neuronal membrane by matrix metalloproteinase 9 (MMP9) and MMP2 and by microglial cell-derived cathepsin S (CatS) and signals via CX3C-chemokine receptor 1 (CX3CR1), which is expressed by microglia. CCR2 and CX3CR1 signalling also induces the expression of integrins (such as intercellular adhesion molecule 1 (ICAM1) and platelet endothelial cell adhesion molecule (PECAM1)) by endothelial cells, allowing the transendothelial migration of T cells. Neuronal cell release of CCL21 stimulates local microglia via CCR7, and infiltrating T cells are activated via CXCR3. ATP induces microgliosis via the purinergic receptors P2X3R, P2X7R, P2Y12R and P2Y13R. Microglia express Toll-like receptor 2 (TLR2) and TLR4 leading to activation of the MYD88 pathway after detection of endogenous danger signals, such as heat shock protein 60 (HSP60), HSP90, high mobility group box 1 (HMGB1) and fibronectin. Following the detection of such signals, many intracellular pathways are recruited including SRC family kinases (SRC, LYN and FYN), MAPKs (extracellular signal-regulated kinase (ERK), p38 and JUN N-terminal kinase (JNK)) and the inflammasome. This leads to phenotypic changes, increased cell motility and proliferation, altered receptor expression, the activation of transcription factors, such as nuclear factor-κB (NF-κB), and the production of inflammatory mediators (such as interleukin-1β, (IL-1β), IL-18, tumour necrosis factor (TNF) and brain-derived neurotrophic factor (BDNF)). Interleukin-1 receptor (IL-1R1) signalling reduces microglial cell expression of G protein-coupled receptor kinase 2 (GRK2), which is a negative regulator of G protein-coupled receptors (GPCRs), including chemokine receptors. This leads to sustained GPCR signalling, and the exaggerated and prolonged production of pro-inflammatory mediators. The release of soluble mediators also provides a feedback mechanism by which further immune cells are activated.