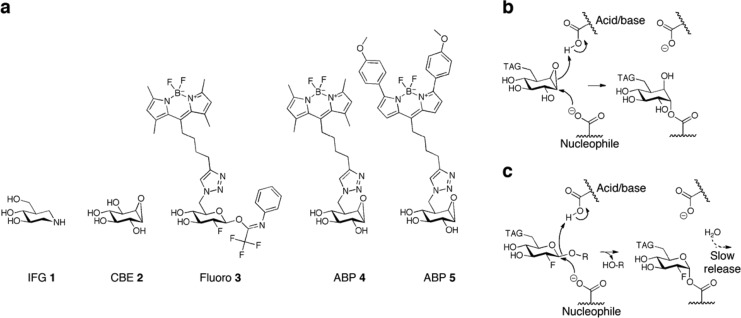
Figure 1.
Inhibitors and reaction mechanism. (a) Structural formulas of competitive, reversible inhibitor isofagomine (IFG 1), irreversible inhibitors conduritol β-epoxide (CBE 2), semi-irreversible inhibitor 2-deoxy-2-fluoro-β-d-glucopyranosyl-N-phenyltrifluoroacetimidate33 (fluoro 3), cyclophellitol β-epoxide type ABP 4 (MDW933, green fluorescent), and β-epoxide-type ABP 5 (MDW941, red fluorescent).35 (b) Irreversible binding mechanism of β-epoxide-type ABPs to the nucleophile of GBA via its double-displacement mechanism. (c) Hydrolysis of fluoro 3 and temporary trapping of the glycosylated nucleophile adducts of GBA.