Summary
Current guidelines in the setting of exposures to potentially rabid bats established by the Advisory Committee on Immunization Practices (ACIP) address post-exposure prophylaxis (PEP) administration in situations where a person may not be aware that a bite or direct contact has occurred and the bat is not available for diagnostic testing. These include instances when a bat is discovered in a room where a person awakens from sleep, is a child without an adult witness, has a mental disability or is intoxicated. The current ACIP guidelines, however, do not address PEP in the setting of multiple persons exposed to a bat or a bat colony, otherwise known as mass bat exposure (MBE) events. Due to a dearth of recommendations for response to these events, the reported reactions by public health agencies have varied widely. To address this perceived limitation, a survey of 45 state public health agencies was conducted to characterize prior experiences with MBE and practices to mitigate the public health risks. In general, most states (69% of the respondents) felt current ACIP guidelines were unclear in MBE scenarios. Thirty-three of the 45 states reported prior experience with MBE, receiving an average of 16.9 MBE calls per year and an investment of 106.7 person-hours annually on MBE investigations. PEP criteria, investigation methods and the experts recruited in MBE investigations varied between states. These dissimilarities could reflect differences in experience, scenario and resources. The lack of consistency in state responses to potential mass exposures to a highly fatal disease along with the large contingent of states dissatisfied with current ACIP guidance warrants the development of national guidelines in MBE settings.
Keywords: Bats, rabies, prophylaxis, human, exposures, guidelines
Introduction
Worldwide, among countries that have eliminated canine rabies, wildlife reservoirs have become an increasingly significant public health burden (Wallace et al., 2014). In the United States, bats account for 29% of all reported rabid animals, and rabies is enzootic in bats in all continental states (Messenger et al., 2002, Centers for Disease and Prevention, 2013a). Bats play a critical role in rabies infections in humans in the United States, accounting for more than 90% of indigenously acquired human rabies since 1980 (Messenger et al., 2002; Blanton et al., 2006; Patyk et al., 2012). Cryptic rabies deaths associated with bats (i.e. deaths attributed to bats, but for which no exposure was known to have occurred) account for 44–89% of human deaths in the United States since 1950 (Warrell, 1995; Messenger et al., 2002; de Serres et al., 2008; Petersen, 2011). Therefore, public health precautions must be taken when individuals have direct contact or the possibility of direct contact with bats to ensure that potential exposures are recognized and evaluated by a medical professional. With rare exception, rabies infections in humans is always fatal (Willoughby et al., 2005); thus, post-exposure prophylaxis (PEP) remains critical in preventing rabies among individuals of high-risk animal exposures to rabies. The current Advisory Committee on Immunization Practices (ACIP) recommends a PEP regimen which entails administration of human rabies immunoglobulin (HRIG) followed by administration of rabies vaccine doses on days 0, 3, 7 and 14 in a previously unvaccinated, exposed person without immunosuppressive conditions (Rupprecht et al., 2010).
ACIP provides recommendations for the evaluation and vaccination of a single person–single bat or bat colony exposure (Manning et al., 2008). In this setting, ACIP recommendations state that persons with the following conditions may qualify for rabies PEP: instances in which a bat is discovered in a room where a person is a child without an adult witness, has a mental disability, or is intoxicated or otherwise impaired. However, not as clearly stated are recommendations for circumstances in which numerous persons are potentially exposed to one or more bats during the same event, or in instances where numerous people have been exposed to a bat colony over an extended period of time. The lack of clarity regarding public health’s response to mass bat exposure (MBE) events has led to inconsistent public health responses ranging from a ‘vaccinate everyone’ approach to approaches in which triage systems are employed in order to identify the subset of persons whom are determined to meet situation-specific exposure criteria for PEP (Centers for Disease and Prevention, 2013a, Webber et al., 2014). A PEP regimen can be costly, ranging from $1634 to 8415 including hospital and physician charges (Dhankhar et al., 2008). In situations where large numbers of individuals may have been exposed to a bat or bat colony, administration of PEP in the absence of establishing exposure risk criteria could result in excessive and needless expenditures. Minor adverse reactions, such as malaise, fever and pain at the injection site, are common with the rabies vaccination series, and severe adverse reactions, while extremely rare, are possible. In addition, unnecessary use of rabies vaccine places a burden on rabies vaccine stockpiles (Centers for Disease and Prevention, 2009). Care should be taken to avoid injudicious administration of rabies vaccines (Rupprecht et al., 2010).
The intent of this report is to describe current MBE investigation practices for potential indigenous rabies exposures among state and local public health agencies (PHAs) and identify inconsistencies in approaches to investigation and PEP recommendations. The results from this report may inform local and state health department policies on MBE response and the need for clarification in national recommendations.
Methods
An online 23-question survey to assess a public health jurisdiction’s response, attitudes and burdens in the setting of an indigenous MBE event was developed and distributed to state and local public health veterinarians via email in 2013. The National Association of State Public Health Veterinarians listserv was utilized to identify appropriate state and local public health veterinarians. If a PHA responded that they did not have a designated public health veterinarian, the survey was directed to the infectious disease expert assigned to bat exposures. For the purposes of this study, an MBE event was defined as 10 or more persons that were potentially exposed to a bat or bat colony associated with an acute event. The definition specified ‘acute events’ to avoid describing events that can easily be addressed through an individual health assessment. PHAs with no history of MBE investigations (but limited to only phone inquiries) in the 5 years prior to receiving the survey were categorized as having ‘little or no experience’, while PHAs that have investigated MBE events during the same time period were categorized as having ‘prior experience’.
Emails were sent to representatives in 49 states (Hawaii was excluded because it had no known animal rabies) of the United States and New York City. The survey was designed to assess the respondent’s experiences and practices with MBE events particularly involving the following: investigations and perceived degree of clarity of current recommendations (Appendix 1). Survey responses were multiple choice, open text entry and Likert-type scales of 1–5 (5 being highest). Missing data or responses requiring clarification were followed up with the PHA by email. Missing responses where a PHA did not respond to a follow-up inquiry were excluded from the analysis.
Data were analysed with Microsoft® Excel. Statistical analysis was conducted in Epi Info™ 7 (Centers for Disease Control and Prevention, Atlanta, GA, USA) and responses were considered statistically significant when P < 0.05.
Results
Forty-five of the 50 (90%) PHAs completed the survey (Table 1). Twelve (27%) of the 45 respondents had little or no experience; 33 (73%) respondents reported prior experience with MBE events. Three (25%) of the PHAs with little or no experience and 19 (58%) with prior experience reported a prior history of indigenous human rabies cases associated with bats in their respective jurisdictions. Overall, PHAs with little or no MBE experience received an average of 1.2 calls/year (range 0–10, mode 0), which included phone consultations and inquiries regarding suspected MBE events, compared to 16.9 calls/year among states with prior MBE experience (range 1–300, mode 2). The average reported number of annual investigations which met the MBE definition among PHAs with little or no prior experience versus PHAs with prior MBE experience was 0.3 and 4.7, respectively. Average person-hours invested annually in MBE investigations among PHAs with little or no experience versus PHAs with prior MBE experience were 33.3 and 106.7 person-hours, respectively.
Table 1.
Number of public health agencies that have reported human cases of bat variant rabies, and the number of calls, investigations and person-hours invested for mass bat exposure investigations based on prior mass bat exposure experience
| Little or no experience (N = 12) mean, range, mode |
Prior experience (N = 33) mean, range, mode |
All agencies (N = 45) mean, range, mode |
|
|---|---|---|---|
| Number of agencies reporting prior human cases from bat variant rabies | n = 3 (25%) | n = 19 (58%) | n = 22 (49%) |
| Mass bat exposure consultations per year per agency | 1.17, 0–10, 0 | 16.9, 1–300, 2 | 12.5, 0–300, 2 |
| Investigations of possible mass bat exposures per year per agency | 0.3, 0–1, 0 | 4.7, 0–50, 1 | 3.5, 0–50, 1 |
| Person-Hours per mass bat exposure investigation per year per agency | 33.3, 0–80, 0 | 106.7, 80–400, 80 | 87.1, 0–400, 80 |
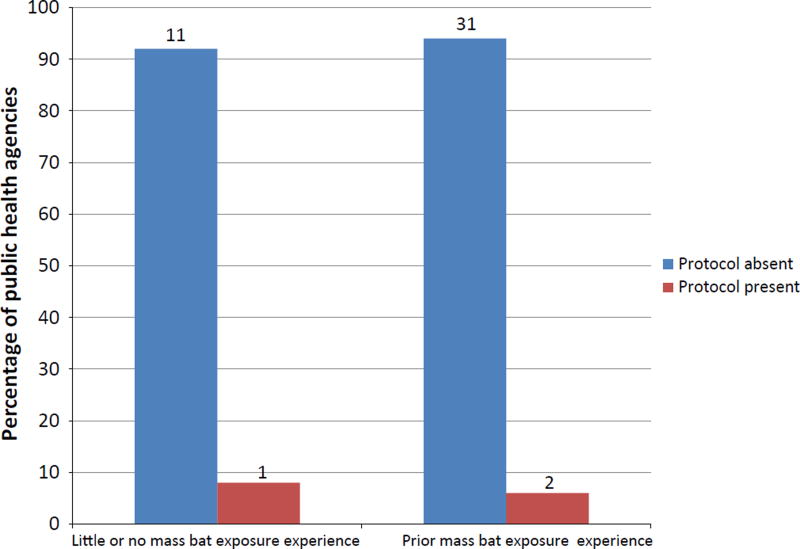
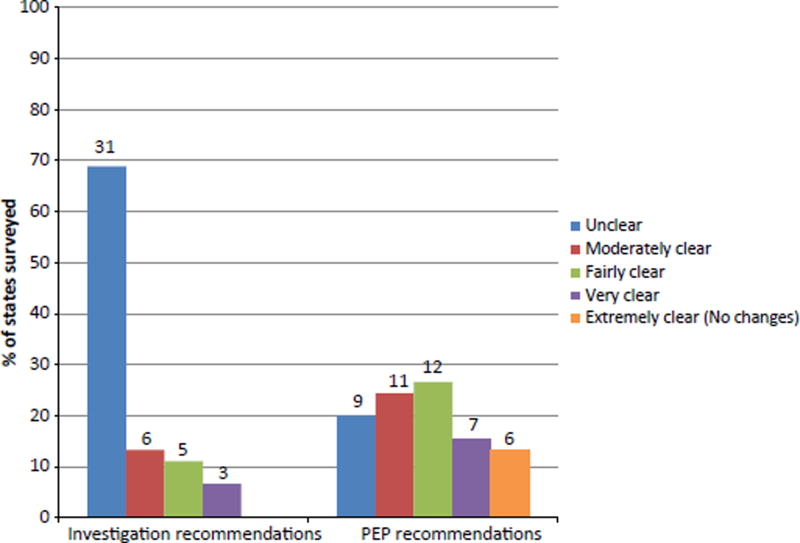
Only three PHAs (7% of respondents) had a protocol in place to respond to an MBE event (Fig. 1). Thirty-one (69%) and 9 (20%) PHAs reported that ACIP recommendations for investigations and PEP in MBE settings, respectively, were unclear and improvements were needed (Fig. 2). Forty-two (93%) and 32 (71%) respondents claimed that ACIP guidelines were less than clear (unclear, moderately clear and fairly clear) in addressing investigations and PEP in MBE settings, respectively. In addition, none of the PHAs reported that ACIP provided clear guideline for MBE investigation, and only 6 (13%) PHAs reported that ACIP was extremely clear in MBE PEP recommendations.
Fig. 1.
The number and percentage of public health agencies (N = 45) and their reported mass bat exposure (MBE) protocol status (absent or present) based on the agency’s prior experience. Little or no MBE experience = agencies reporting no confirmed MBE events in the previous 5 years of survey; prior MBE experience = agencies with MBE event reported in previous 5 years of survey. Number of agencies are reported above each bar.
Fig. 2.
The number and percentage of public health agencies (N = 45) reporting on the clarity of ACIP on investigation recommendations and PEP recommendations in the setting of mass bat exposures. Number of agencies are above each bar.
Public health agencies with prior MBE experience were more likely to engage at least one specialist compared to PHAs with little or no MBE experience. Engagement of an environmental health specialist was statistically significant when comparing the PHA with prior experience to those with little or no experience (OR = 4.6, P = 0.04) (Table 2). PHAs with prior MBE experience were also 1.4 times more likely (94% versus 66%) to engage two or more specialists during MBE investigations (wildlife specialist, animal control, environmental health specialist, CDC). In addition, when asked how issues of PEP compliance among potential MBE patients were handled, PHAs with prior MBE experience were 1.9 times more likely (48% versus 25%) to utilize two or more mechanisms of follow-up to ensure PEP recommendations were adhered (Table 3). PHAs with prior MBE experience were more likely to conduct home visits, send certified letters or require a signed declination in cases where PEP was declined compared to PHAs with little or no MBE experience, but only sending a certified letter (OR = 5.1, P = 0.01) and practicing ‘no follow-up’ (OR = 0.1, P = 0.01) were statistically significant when comparing PHAs with prior MBE experience to PHAs with little or no experience.
Table 2.
Stakeholders whom public health agencies engage during a mass bat exposure investigation
| Little or no experience, n (%) |
Prior experience, n (%) |
OR | P-value | % of all agencies (N= 45) |
|
|---|---|---|---|---|---|
| Wildlife specialist | 9 (75) | 26 (79) | 1.2 | 0.8 | 78 |
| Animal control | 5 (42) | 22 (67) | 2.8 | 0.1 | 60 |
| Environmental health specialist | 3 (25) | 20 (61) | 4.6 | 0.04 | 51 |
| Centers for disease control and prevention | 5 (42) | 21 (64) | 2.5 | 0.2 | 58 |
| Number of agencies that engage 0 specialty groups | 2 (17) | 1 (3) | 6.4 | 0.1 | 7 |
| Number of agencies that engage only 1 specialty group | 2 (17) | 1 (3) | 7 | ||
| Number of agencies that engage only 2 specialty groups | 5 (42) | 9 (27) | 31 | ||
| Number of agencies that engage only 3 specialty groups | 2 (17) | 16 (49) | 40 | ||
| Number of agencies that engage all 4 specialty groups | 1 (7) | 6 (18) | 15 |
Table 3.
How public health agencies report having responded to individuals who refuse PEP in the setting of a mass bat exposure
| Little or no experience, n (%) |
Prior experience, n (%) |
OR | P-value | % of all agencies (N = 45) |
|
|---|---|---|---|---|---|
| Home visit | 4 (33) | 13 (39) | 1.3 | 0.7 | 38 |
| Certified letter | 4 (33) | 24 (73) | 5.1 | 0.01 | 62 |
| Signed declination form | 2 (17) | 8 (24) | 1.6 | 0.6 | 22 |
| Deferred or no follow-up | 5 (42) | 3 (9) | 0.1 | 0.01 | 18 |
| Othera | 2 (17) | 8 (24) | 1.6 | 0.6 | 22 |
| Number of agencies that respond using 0 mechanismsb | 4 (33) | 3 (9) | 5 | 0.05 | 16 |
| Number of agencies that respond using 1 mechanismc | 5 (42) | 14 (42) | 42 | ||
| Number of agencies that respond using 2 mechanismsc | 2 (17) | 9 (27) | 24 | ||
| Number of agencies that respond using 3 mechanismsc | 1 (8) | 6 (18) | 16 | ||
| Number of agencies that respond using 4 mechanismsc | 0 (0) | 1 (3) | 2 |
‘Other’ includes uncertified letter, follow-up with phone call, court order, CPS.
Includes only ‘deferred or no follow-up’.
Excludes the states that only have ‘deferred or no follow-up’ as a response.
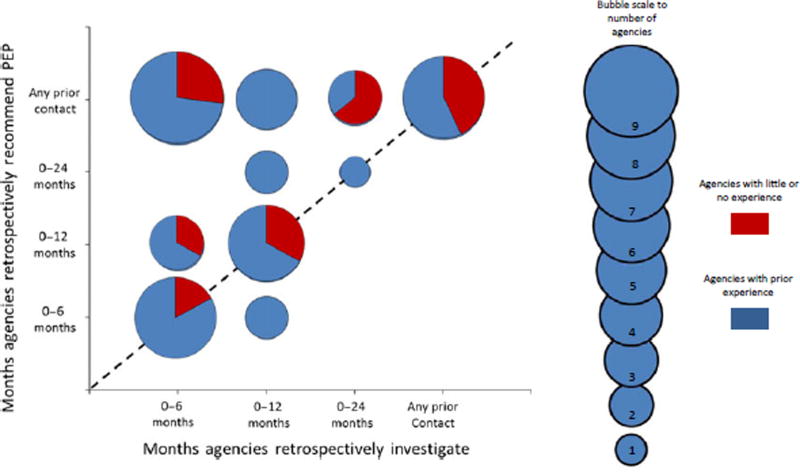
Responses regarding the timing of a MBE investigation and administration of PEP retrospectively (i.e. how far back the PHA was willing to administer PEP from time of known rabies exposure) were evaluated based on agencies’ prior experience with MBE. Responses to these questions regarding the retrospective investigation and PEP policies were plotted on a bubble graph (Fig. 3). The dashed line represented equal retrospective time frames for MBE event investigation and PEP administration (i.e. a PHA will investigate exposures to a bat colony for a 12-month retrospective time period and will recommend PEP to anyone found to have an exposure during that 12-month period). Plots above the dashed line represented PHAs whose time frames for PEP administration were longer (or more retrospectively) than the time frames over which the PHA would conduct an MBE investigation (i.e. a PHA will investigate exposures to a bat colony for a 6-month retrospective time period, but would have recommended anyone with an exposure in the past 12 months to be vaccinated). Likewise, PHAs plotted below the dashed line represented PHAs whose time frames for PEP administration were shorter (less retrospective) than the time frame for which they would conduct an MBE investigation (i.e. a PHA will investigate exposures to a bat colony that have occurred in the past 12 months, but would only recommend PEP for exposures identified within the past 6 months). Twenty (44%) PHAs recommended PEP over the same retrospective time frame as the investigation. Twenty-three (51%) PHAs recommended PEP during a longer retrospective time frame compared to the time frame in which they would conduct an MBE investigation (Fig. 3). Furthermore, 18 (40%) PHAs recommended PEP regardless of how far in the past the exposure occurred. Only two (4%) PHAs recommended PEP in a time frame that was less than the MBE investigation time frame. For example, during the course of an MBE investigation, PEP administration was recommend for bat contact occurring in the past 6 months, but the investigation would assess people for exposures within the past 12 months. Overall, PHAs with little or no MBE experience either recommended PEP within the same time frame or over a longer retrospective period than the investigation.
Fig. 3.
Bubble graph comparing the months public health agencies (N = 45) are willing to administer PEP prior to suspected bat exposure versus the months public health agencies are willing to investigate an MBE event prior to suspected bat exposure. Bubbles that fall on the dashed lines represent agencies that retrospectively recommend PEP and investigate MBE events within the same time frame. Bubbles that are above the dashed line are agencies that are more likely to recommend PEP beyond the time span of a retrospective investigation while bubbles that fall under the dashed line are agencies that are less likely to recommend PEP during the time span of a retrospective investigation. Bubble area correlates with the number of agencies. Red = agencies with little or no experience, Blue = agencies with prior experience.
Discussion
Mass bat exposure events pose a significant and unique public health concern, not only because of the high fatality of rabies, but also because of the potential for numerous human exposures that must be investigated, assessed and appropriately treated. Although expensive, rabies PEP is extremely effective when administered appropriately before symptoms develop; however, excessive PEP administration in MBE settings is generally not recommended (Rupprecht et al., 2010). Thus, balancing the cost-effectiveness of PEP administration with what is considered ‘best public health practice’ is a challenge.
When considering perceptions and practices of PHAs confronted with MBE scenarios, most PHAs did not have an MBE-specific protocol in place to guide investigations and PEP recommendations. Findings from this study suggest that PHAs with little or no prior MBE experience may not be prepared for large-scale investigations that are often required for MBE events. During an investigation, PHAs with little or no MBE experience are less likely to engage experts in the investigation, particularly environmental health specialists, who can play critical roles in safety assessments and appropriate bat removal efforts (Centers for Disease and Prevention, 2013a). PHAs with prior MBE experience were in fact more likely to engage specialists compared to PHAs with little or no experience. This suggests that PHAs with more experience valued the important roles of experts in conducting thorough MBE investigations and were probably more capable of distributing the workload associated with MBE investigations. Similarly, in instances where individuals refused PEP, PHAs with prior MBE experience were more likely to follow up with an individual in a formal manner, such as by certified letter, while PHAs with little or no MBE experience were less likely to take any actions to ensure PEP recommendations were followed. While PHAs with prior MBE experience more often reported active investigation techniques as described above, the reported actions by PHAs varied greatly. These findings indicate that PHA responses to MBE investigations are directed more by prior experience with MBE events, rather than by reliance upon existing ACIP recommendations. This could explain why among 33 PHAs with prior experience with MBE events, 31 did not have an established protocol in place (Fig. 1). Robust national guidelines could provide a framework to guide appropriate PHA responses to MBE events, which is not only greatly needed in PHAs with little or no MBE experience, but could benefit all PHAs.
The ACIP is the only national recommendation document to guide healthcare-related decisions regarding rabies exposures. This document addresses the public health response when a person is potentially exposed to a bat. However, the existing ACIP recommendations do not address the extensive public health approaches that may be undertaken during an MBE event. This survey asked two questions relating to ACIP guidelines in regard to MBE events: clarity of recommended investigation practices and clarity of PEP recommendations. Most PHAs found the ACIP guidelines less than clear in guiding informed PEP decisions in relation to MBE. An explanation for this is that ACIP recommendations were largely designed with respect to PEP for a single person–single bat exposure, rather than numerous persons with often obscure exposure scenarios that must be assessed by public health practitioners. An even greater majority of PHAs indicated that guidelines for investigating MBE events were lacking and needed improvements in clarity. MBE events are often identified when a bat colony is discovered in a public or communal setting, such as in an apartment complex, a vacation rental or a summer camp. Historically, PHA investigations within such settings have often spanned many months or even years in order to identify persons who may have been exposed (Centers for Disease and Prevention, 2009, 2013a; Webber et al., 2014). These are details which are not currently discussed in any recommendation document, and the findings from this survey support the development of a guidance document for PHAs to consult when faced with an MBE event.
It was striking that more than 50% of PHAs, regardless if they reported MBE experience or not, were not consistent in their recommendation for MBE investigation and PEP administration. To minimize PEP costs and person-hour investments in an investigation, one would assume that it is standard practice to administer PEP within the same time frame as the MBE investigation. In fact, numerous PHAs reported they would provide PEP for any bat exposure, irrespective of how far in the past the exposure occurred, indicating that PEP practices in MBE settings are not clear for many PHAs. The typical incubation period for bat-variant rabies virus is 3 weeks to 3 months, with no known reports of cases with an incubation period longer than 12 months (de Serres et al., 2008; Carrara et al., 2013; Udow et al., 2013). Therefore, there is currently no evidence to support the recommendation of PEP for persons reporting an exposure greater than 12 months prior. In contrast, some PHAs also reported they would investigate MBE events for a longer retrospective time frame compared to the time frame for which they would recommend PEP. For instance, two PHAs indicated that they would investigate MBE events that occurred up to 12 months prior; however, they would only provide PEP for persons with an exposure(s) in the past 6 months. This leaves a question as to what public health recommendations were made to persons exposed greater than 6 months prior, and why resources were expended to investigate MBE events during time periods in which no public health action would take place.
While no multihuman rabies deaths caused by an MBE event have ever been documented in the United States, there have been published MBE events that would have met this study’s MBE definition. For example, in 2004, a 15-year-old girl was bitten by a bat while attending church (Centers for Disease and Prevention, 2004). This event involved numerous persons in the same room as a bat, with at least one confirmed human exposure, but was not reported to health officials. Timely reporting of the exposure could have spurred a thorough investigation. Furthermore, in two other human rabies cases, involving a 46-year-old woman and a 77-year-old woman, each of the victims resided in homes which were colonized by bats, had multiple reported bat sightings in living spaces and had frequent visitors inside of the homes. Per this study’s MBE definition (multiple persons exposed to a bat or bat colony), both scenarios would have been considered MBE events (Centers for Disease and Prevention, 2013b, Harrist et al., 2016). Although in one of these three cases, the PHA was not alerted of the MBE in a timely manner, this highlights the importance of developing national guidelines in tandem with educational activities to ensure the public and PHAs are equally aware of the critical importance of timely exposure reporting. These events also show that human deaths have occurred in the setting of MBE events which should be further public health precedence to develop MBE guidelines. A goal of public health is to practice due diligence in providing guidance and tools so such catastrophic events will never occur. We would therefore argue that a pro-active approach in establishing such guidelines can only be beneficial to PHAs when challenged with an MBE event.
There were various limitations in this study. The survey was distributed to PHAs with the intention of targeting state veterinary epidemiologists; however, several PHAs did not have a state veterinary epidemiologist so the survey often was deferred to the infectious disease epidemiologist in the agency. Regardless of background, results could have been affected by the perspective and level of rabies experience of the survey responder. Also, responses to the survey could be biased by recall. For instance, the survey response to questions of the number of MBE investigations and calls received annually may be influenced by how long the responder has been employed at that PHA. A survey responder who was employed within the previous 2 or 3 years may not comprehend the true number of MBE investigations in the previous 5 years (e.g. some states reported up to 50 MBE investigations per year and 400 person-hours dedicated to MBE investigations). Unfortunately, the survey did not ask how long the responder had been employed in the position to address this issue.
The findings from this study support the need for either development of MBE-specific guidelines or clarification of current national guidelines for public health investigations and PEP recommendations in the setting of MBE events. MBE guidelines should address elements necessary for a full-scale investigation and the appropriate time frame for retrospective investigation of a reported event. Guidelines could include scenario-specific MBE tools, with the understanding that scenarios are not rigid and the tools should therefore be adaptable to the specific setting. A clear set of guidelines could help direct resources where they are most needed so that PHA burdens are reduced and to ensure that all critical components of an MBE response are addressed. Because of the challenges and complexity of creating such guidelines, they should be developed through collaborations with local, state and federal rabies experts, specifically those with MBE experience. For this reason, an MBE working group, consisting of CDC and state bat rabies experts, has been formed with the specific task of developing tools and guidelines for MBE investigations. Through continual meetings and collaboration, the working group not only aims to achieve these goals but to disseminate the information to PHAs expeditiously. Although the ACIP recommendations may not provide explicit protocols for MBE events, the single person– single bat exposure assessment criteria can be extrapolated and refined for use in MBE events. In conclusion, any MBE guidelines should be clear, thorough and should accommodate variations and differences in approach between PHAs and in the complexities of individual MBE events.
Impacts.
Rabies, a highly fatal infection, is most often caused by bat exposures in the United States. Current guidelines on human rabies caused by bats are explicit only for one-bat one-human exposures but not mass bat exposures (MBEs) (10 or more humans exposed to a bat or bat colony).
A survey among U.S. public health agencies found wide variations in practices and perceptions of MBE and a general consensus that national guidelines were needed.
National guidelines for MBEs could better define usage of post-exposure prophylaxis with the hope of limiting its administration to high-risk exposures and potentially reduce costs of treatment, save on prophylaxis stockpile and decrease the risk of adverse events associated with prophylaxis.
Appendix 1. Survey distributed to state and local public health agencies to assess perceptions and practices of mass bat exposure in the setting of rabies
Attitudes and Practices of State Public Health Veterinarians on Investigation of Potential Mass Rabies Exposures Due to Bats
Instructions
The CDC Rabies Program, in collaboration with the National Association of State Public Health Veterinarians, is attempting to evaluate the burden of investigating mass human exposures to bats on the public health system, and current strategies used during these investigations. This questionnaire will aide in the development of guidelines for investigation of mass human exposures to bats.
For the purposes of this questionnaire, mass human exposures to bats refer to an investigation in which it was found that 10 or more persons from multiple families or dwellings were potentially exposed to a bat or a bat colony, associated with an acute event.
We thank you for taking the time to answer these questions.
* Required
What state are you reporting for? *
-
Has your state ever had a bat variant human case of rabies that was acquired in the USA? *
Yes
No
-
Does your state distribute PEP from state stockpiles? *
Yes
No
Unsure
On average, how many people in your jurisdiction receive PEP, annually (if number is unknown, please provide an estimate)? *
On average, how many calls do jurisdictions in your state receive regarding potential mass exposures to bats, annually? *
-
Over the past 5 years has your health department investigated any reports of exposures to bats involving 10 or more persons from multiple families or dwellings, associated with an acute event? *
Yes, and we are willing to provide details on these investigations
Yes, but details for these investigations are not available
No
Other:
Approximately how many investigations are conducted in your state in response to reports of potential mass human exposures to bats, annually? *
-
On a scale of 1 to 5, how would you quantify the total burden (in terms of all local and state staff TIME and NON-FISCAL PROGRAM RESOURCES) associated with your jurisdictions ANNUAL ‘mass human exposure to bats’ investigations? *
1 = Minimal staff time, few program resources, no interruption of normal daily duties
2
3
4
5 = High burden on staff and funding, integral services are disrupted during investigations
Other:
-
On a scale of 1 to 5, how would you quantify the total burden (in terms of all local and state PROGRAM FUNDS) associated with your jurisdictions ANNUAL ‘mass human exposure to bats’ investigations? *
1 = Little program funds typically used for investigations
2
3
4
5 = Investigations often require large amounts of programs funds, resulting in disruption of other core activities
Other:
-
In your average year, approximately how many person-hours does your program dedicate to investigation and follow-up on potential contacts of a mass human exposure to bats investigation? *
(please answer in terms of ONE average investigation, not an annual cumulative calculation)
0 person-hours
1–80 person-hours
81–160 person-hours
161–400 person-hours
400+ person-hours
-
During investigations of mass human exposures to bats do you engage: * (select all that apply)
Wildlife specialists in bat exclusion Animal Control officers
Environmental Health Specialists (e.g. Industrial Hygienists)
CDC Personnel (either remotely or in person)
Private pest control companies
Other:
-
Does your state/jurisdiction have an existing investigation protocol for mass human exposures to bats? *
Yes
No
Other:
-
If yes, are you willing to share the protocol? *
Yes
No
Not Applicable
Other:
-
On a scale of 1 to 5, how clear are current national recommendations on proper investigative techniques in response to mass human exposures to bats? *
1 = not clear, improvements needed
2
3
4
5 = very clear, no improvements in recommendations needed
Unaware of any national recommendations for investigating mass human exposure to bats
-
On a scale of 1 to 5, how clear are current ACIP recommendations on the appropriate administration of PEP after bat exposure, in the setting of mass human exposure to bats? *
1 = not very clear, improvements needed
2
3
4
5 = very clear, no improvements in recommendations needed
-
During investigations of mass human exposures to bats, do you recommend that all persons reporting that they were in the same room with a bat receive PEP? *
If ‘Maybe’ or ‘unknown’, please fill in explanation in ‘Other’ field.
Yes
No
Other:
-
During investigations of mass human exposures to bats, are PEP recommendations in your state based on the exposed person’s age, mental status, drug use or sleeping disorders? *
If ‘Maybe’ or ‘unknown’, please fill in explanation in ‘Other’ field.
Yes
No
Other:
-
During an investigation of mass human exposures to bats, how far in the past would you consider investigating potential exposures, if it was found out that bats had been in the dwelling for many years? *
0–6 months
0–12 months
0–24 months
As long as bats were reported in the dwelling
-
During an investigation, how far in the past would you consider that a known bite or scratch from a bat still indicates a need for PEP? *
0–6 months
0–12 months
0–24 months
Any prior bat contact (bite or scratch) warrants PEP (i.e. greater than 2 years)
-
Does your jurisdiction have seasonal restrictions on bat removal from dwellings? *
If ‘Maybe’ or ‘unknown’, please fill in explanation in ‘Other’ field.
Yes
No
Other:
-
Does your jurisdiction permit exemptions for bat removal from dwellings when there is a public health concern? *
If ‘Maybe’ or ‘unknown’, please fill in explanation in ‘Other’ field.
Yes
No
Other:
-
What steps does your jurisdiction take if a person refuses PEP? * Select all that apply:
Home visits
Certified Letters (return receipt) from Local Health Director or Public Health Veterinarian Declination forms
No follow-up
Other:
-
When considering other public health issues you routinely address, how concerned are you about establishing better recommendations for the investigation of mass human exposures to bats? *
1 = not concerned about establishing recommendations
2
3
4
5 = very concerned about the need for new recommendations
References
- Blanton JD, Krebs JW, Hanlon CA, Rupprecht CE. Rabies surveillance in the United States during 2005. J. Am. Vet. Med. Assoc. 2006;229:1897–1911. doi: 10.2460/javma.229.12.1897. [DOI] [PubMed] [Google Scholar]
- Carrara P, Parola P, Brouqui P, Gautret P. Imported human rabies cases worldwide, 1990–2012. PLoS Negl. Trop. Dis. 2013;7:e2209. doi: 10.1371/journal.pntd.0002209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centers for Disease C. & Prevention. Recovery of a patient from clinical rabies–Wisconsin, 2004. MMWR Morb. Mortal Wkly Rep. 2004;53:1171–1173. [PubMed] [Google Scholar]
- Centers for Disease C. & Prevention. Human exposures to a rabid bat – Montana, 2008. MMWR Morb. Mortal. Wkly Rep. 2009;58:557–561. [PubMed] [Google Scholar]
- Centers for Disease C. & Prevention. Assessment of risk for exposure to bats in sleeping quarters before and during remediation - Kentucky, 2012. MMWR Morb. Mortal. Wkly Rep. 2013a;62:382–384. [PMC free article] [PubMed] [Google Scholar]
- Centers for Disease C. & Prevention. Human rabies–South Carolina, 2011. MMWR Morb. Mortal. Wkly Rep. 2013b;62:642–644. [PMC free article] [PubMed] [Google Scholar]
- Dhankhar P, Vaidya SA, Fishbien DB, Meltzer MI. Cost effectiveness of rabies post exposure prophylaxis in the United States. Vaccine. 2008;26:4251–4255. doi: 10.1016/j.vaccine.2008.05.048. [DOI] [PubMed] [Google Scholar]
- Harrist A, Styczynski A, Wynn D, Ansari S, Hopkin J, Baker J, Nakashima A, Atkinson A, Spencer M, Dean D, Teachout L, Mayer J, Petersen B, Wallace R, Musgrave K. Human Rabies - Wyoming/Utah, 2015. MMWR Morb. Mortal. Wkly Rep. 2016;65:529–533. doi: 10.15585/mmwr.mm6521a1. [DOI] [PubMed] [Google Scholar]
- Manning SE, Rupprecht CE, Fishbein D, Hanlon CA, Lumlertdacha B, Guerra M, Meltzer MI, Dhankhar P, Vaidya SA, Jenkins SR, Sun B, Hull HF Advisory Committee on Immunization Practices Centers for Disease C. & Prevention. Human rabies prevention–United States, 2008: recommendations of the Advisory Committee on Immunization Practices. MMWR Recomm. Rep. 2008;57:1–28. [PubMed] [Google Scholar]
- Messenger SL, Smith JS, Rupprecht CE. Emerging epidemiology of bat-associated cryptic cases of rabies in humans in the United States. Clin. Infect. Dis. 2002;35:738–747. doi: 10.1086/342387. [DOI] [PubMed] [Google Scholar]
- Patyk K, Turmelle A, Blanton JD, Rupprecht CE. Trends in national surveillance data for bat rabies in the United States: 2001–2009. Vec. Bor. Zoonot. Dis. 2012;12:666–673. doi: 10.1089/vbz.2011.0839. [DOI] [PubMed] [Google Scholar]
- Petersen BARC. Human rabies epidemiology and diagnosis. In: Tkachev S, editor. Non-Flavivirus Encephalitis. InTech, Rijeka; Croatia: 2011. pp. 1–32. Ch. 11. [Google Scholar]
- Rupprecht CE, Briggs D, Brown CM, Franka R, Katz SL, Kerr HD, Lett SM, Levis R, Meltzer MI, Schaffner W, Cieslak PR Centers for Disease C. & Prevention. Use of a reduced (4-dose) vaccine schedule for postexposure prophylaxis to prevent human rabies: recommendations of the advisory committee on immunization practices. MMWR Recomm. Rep. 2010;59:1–9. [PubMed] [Google Scholar]
- de Serres G, Dallaire F, Cote M, Skowronski DM. Bat rabies in the United States and Canada from 1950 through 2007: human cases with and without bat contact. Clin. Infect. Dis. 2008;46:1329–1337. doi: 10.1086/586745. [DOI] [PubMed] [Google Scholar]
- Udow SJ, Marrie RA, Jackson AC. Clinical features of dog- and bat-acquired rabies in humans. Clin. Infect. Dis. 2013;57:689–696. doi: 10.1093/cid/cit372. [DOI] [PubMed] [Google Scholar]
- Wallace RM, Gilbert A, Slate D, Chipman R, Singh A, Cassie W, Blanton JD. Right place, wrong species: a 20-year review of rabies virus cross species transmission among terrestrial mammals in the United States. PLoS One. 2014;9:e107539. doi: 10.1371/journal.pone.0107539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warrell MJ. Human deaths from cryptic bat rabies in the USA. Lancet. 1995;346:65–66. doi: 10.1016/s0140-6736(95)92106-0. [DOI] [PubMed] [Google Scholar]
- Webber BJ, Ayers KJ, Winterton BS, Yun HC, Cropper TL, Foster J, Jr, Kren MC, Meek BY, Oliver TA, Hudson CM. Centers for Disease C. & Prevention. Assessment of rabies exposure risk in a group of U.S. Air Force basic trainees - Texas, January 2014. MMWR Morb. Mortal. Wkly Rep. 2014;63:749–752. [PMC free article] [PubMed] [Google Scholar]
- Willoughby RE, Jr, Tieves KS, Hoffman GM, Gha-nayem NS, Amlie-Lefond CM, Schwabe MJ, Chusid MJ, Rupprecht CE. Survival after treatment of rabies with induction of coma. N. Engl. J. Med. 2005;352:2508–2514. doi: 10.1056/NEJMoa050382. [DOI] [PubMed] [Google Scholar]