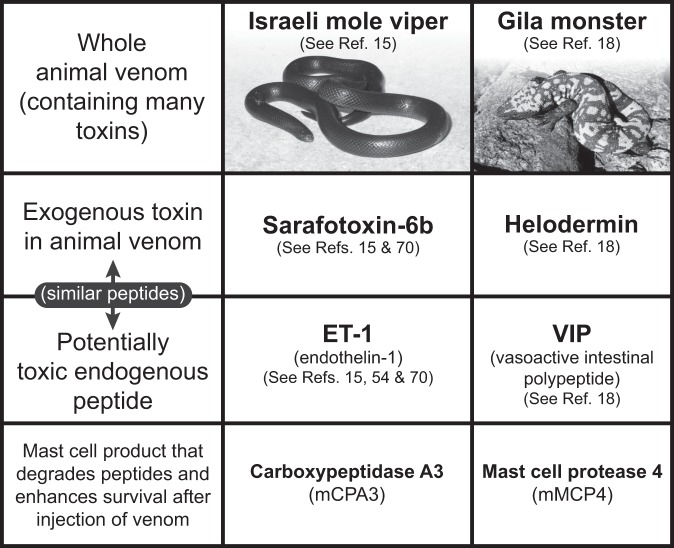
Fig. 3.
Mast cells can enhance innate resistance to high levels of endogenous peptides and structurally similar peptides in reptile venoms. Mast cell cytoplasmic granules contain proteases such as carboxypeptidase A3 (CPA3 [mCPA3 = mouse CPA3]) and mast cell protease 4 (MCP4 [mMCP4 = mouse MCP4]) that, upon secretion by activated mast cells, can degrade certain endogenous peptides, such as endothelin-1 (ET-1) and vasoactive intestinal polypeptide (VIP), respectively, as well as structurally similar peptides contained in the venoms of poisonous reptiles, such as sarafotoxin 6b in the venom of the Israeli mole viper (Atractaspis engaddensis) and helodermin in the venom of the Gila monster (Heloderma suspectum). The ability of mast cells to be activated to degranulate by components of venoms such as these, which can act at the same receptors which recognize the corresponding structurally similar endogenous peptides, permits mast cells to release proteases that can reduce the toxicity of these peptides and which help to enhance the survival of mice injected with the whole venoms of these reptiles, that contain many toxins in addition to sarafotoxin 6b and helodermin. This mechanism may also permit mast cells to restore homeostasis in settings associated with markedly increased levels of the endogenous peptides. [This is a reproduction, in modified form, of Figure 4 from Galli SJ. Rous-Whipple Award Lecture. The mast cell-IgE paradox: from homeostasis to anaphylaxis. Am J Pathol 2016;186:212-24 (reference 42), reprinted with the permission of the publisher, Elsevier, for the American Society for Investigative Pathology.]