Abstract
Biomechanics of the hip joint describes how the complex combination of osseous, ligamentous, and muscular structures transfers the weight of the body from the axial skeleton into the appendicular skeleton of the lower limbs. Throughout history, several biomechanical studies based on theoretical mathematics, in vitro, in vivo as well as in silico models have been successfully performed. The insights gained from these studies have improved our understanding of the development of mechanical hip pathologies such as osteoarthritis, hip fractures, and developmental dysplasia of the hip. The main treatment of end-stage degeneration of the hip is total hip arthroplasty (THA). The increasing number of patients undergoing this surgical procedure, as well as their demand for more than just pain relief and leading an active lifestyle, has challenged surgeons and implant manufacturers to deliver higher function as well as longevity with the prosthesis. The science of biomechanics has played and will continue to play a crucial and integral role in achieving these goals. The aim of this article, therefore, is to present to the readers the key concepts in biomechanics of the hip and their application to THA.
Keywords: Total hip arthroplasty, biomechanics, metal-on-metal, metal on poly, cemented THA, uncemented THA, low friction arthroplasty
MeSH terms: Arthroplasty, replacement, hip, biomechanics, osteoarthritis
Introduction
With one in four people at risk of developing symptomatic hip osteoarthritis in their lifespan, the need for an adequate treatment to address this disabling condition has always been high.1 In the late 19th century, the first attempt at a surgical solution took place through experimental interposition of various tissues (e.g., fascia lata, skin, or even pig's bladder) between the articulating surfaces of the hip.2 After several unfruitful attempts with even glass and ivory as an interface, Philip Wiles3 finally developed the first prosthetic total hip arthroplasty (THA) in 1938 using a metal-on-metal bearing. Unfortunately, his records were lost during the Second World War. McKee, a trainee of Philip Wiles, resumed the development of an uncemented metal-on-metal implant that faced problems of loosening and subsequent mechanical failure. The next-generation cemented McKee-Farrar THA in 1960 was the first successful widely adopted THA.4 Around the same time in another part of northwest UK, another British surgeon, Sir John Charnley was working hard on the concept of a low-friction arthroplasty. During the 1950s, he first experimented with an unsuccessful resurfacing design based on Teflon bearings after which he gradually evolved to the successful cemented arthroplasty design with a metal-on-polyethylene bearing. Facing the competition by McKee and Ring's type metal-on-metal THA in the 1970s, Charnley decided to demonstrate the superiority of the low-friction concept by setting up the famous pendulum experiment comparing the resistance by friction of each prosthesis. His low-friction arthroplasty won the experiment by far and as such it became the most popular THA at that time.
Nowadays, with over 83,000 primary hip joint replacements performed annually within the British NHS system and a reported 78% survival at 35 years of followup, THA is valued as one of the most successful orthopedic interventions of the 20th century.5,6 The development of the ideal THA has been a tale of trial and error, and until now, research has been focusing on improving the design, material and implantation techniques of THA. Clearly, this has been possible because of an intricate and historical relationship between biomechanics of the hip and design of the prosthesis. The aim of the current review, therefore, is to summarize the key concepts in biomechanics of the hip and their application to THA.
The First Bone Law
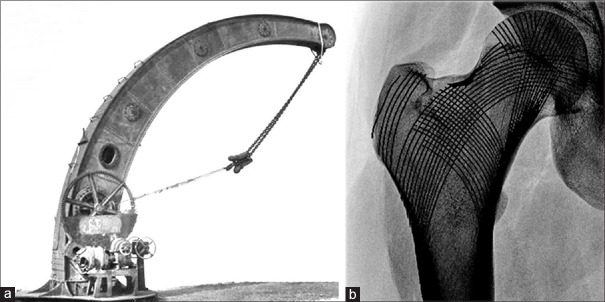
The history of hip joint biomechanics kick started in 1870, when a German surgeon, Julius Wolff, pioneered the mother of all bone laws stating that bone adapts to the loads it is being exposed to.7 Wolff based his concept of the functional form of bone on the similarity between the inner structure of the proximal femur and the lines of internal stress observed in the Fairbairn steam crane [Figure 1]. Previously, the innovative crane, designed by William Fairbairn in 1850, had been the subject of a profound mechanical analysis by the Zurich mathematician, Karl Culmann. At the same time, a professor of anatomy, Georg Hermann von Meyer, was studying the inner trabecular architecture of human bones. Both researchers met in 1966 and together they discovered the similarities between the calculated stress trajectories in the Fairbairn crane and the arched patterns of trabecular bone in the proximal femur. However, it was Wolff who not only acknowledged the similarities discovered by Culmann and von Meyer, but also hypothesized about the adaptive characteristics of bone when subjected to loading.8
Figure 1.
(a) Fairbairn steam crane (b) inner trabecular architecture in the proximal femur
Wolff's theory has a direct application to the design of THA. The femoral stems that bypass the proximal femur and transfer loads directly to the cortical bone at the distal end of the prosthesis will cause stress shielding [Figure 2]. This process gradually results in bone resorption of the bypassed proximal femur and cortical thickening of the loaded distal cortex. Historical femoral stems made of solid metal are characterized by a high overall stiffness. The stiffness of the implant can be lowered by changing the design, as well as selecting materials with a lower elastic modulus such as titanium. Reduction of the implant stiffness at the metaphyseal region with the purpose of approaching the stiffness of bone has proven to be successful in limiting metaphyseal stress shielding.9,10,11 Furthermore, the addition of bioceramics (tricalcium phosphate) to proximal hydroxyapatite-coated stems has showed a modest reduction in the loss of proximal bone mineral density.12 In contrast, design alterations in distal stem shape such as cutouts, flutes, or clothespins have not been proven to avoid any stress shielding.13 Intuitively, the preservation of proximal bone stock is desirable as implant support and is beneficial in the longer run, should periprosthetic fractures occur or revision surgery be required. However, it is important to note that until now there have been no reports on spontaneous fractures or adverse clinical outcomes in the presence of stress-related bone resorption.14,15
Figure 2.
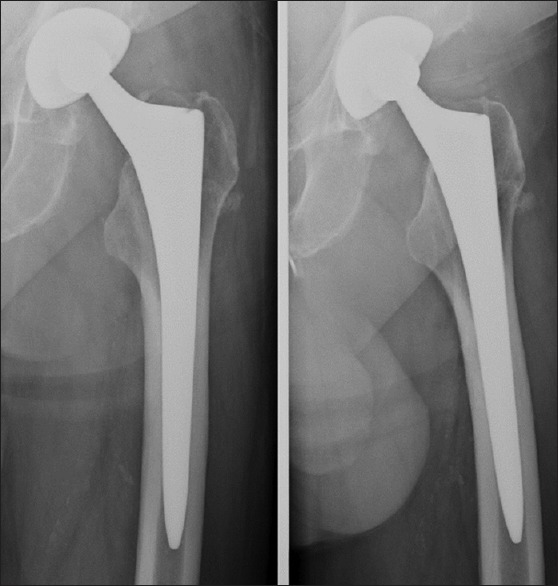
Stress shielding in a left uncemented femoral implant. Note the distal cortical thickening around the canal filling stem and resorption in the metaphyseal Gruen zones 1 and 7
The Static Biomechanical Concept
The biomechanical foundation laid by Wolff and Culmann was further elaborated by Koch16 in 1917 when he published his reference work on the predicted loading of the femur during gait. His calculations assumed a load of 100 lbs directed on the femoral head. He concluded that this load generated compressive forces along the medial side of the femur and both femoral condyles, whereas tensile forces were present on the lateral side. Furthermore, he stated that the lever arm of the bodyweight (BW) compared with the abductor lever arm was twice as long during unipodal stance, which means that the abductor force required to maintain an equilibrium must be twice that of the body's weight. Even though Koch's model was static and did not account for the effect of surrounding muscles on the loaded femur, it stood unchallenged as a reference model of hip biomechanics for the next 50 years.
Koch's model identified the inward varus stress during unipodal stance and assumed the action of the gluteus medius muscle to counterbalance this. Due to the unfavorable abductor lever arm, a theoretically high amount of force and thus high metabolic effort is required during gait. Therefore, Friedrich Pauwels17 introduced his updated biomechanical model in 1976; this included the iliotibial band functioning as a tension band which effectively transformed a part of the lateral distracting force into compressive force and additionally supported the abductor function. The static biomechanical model [Figure 3] describes loading in the hip joint during the unipodal stance phase of normal gait. The model assumes a BW lever arm that is approximately three times the size of the abductor lever arm. Balancing the weight (total BW − the weightbearing leg), therefore, requires an abductor force that is three times the size of the BW. The vectoral sum of the forces acting on the femoral head (reaction force, FR) results in four times the partial body or approximately three times the total BW.
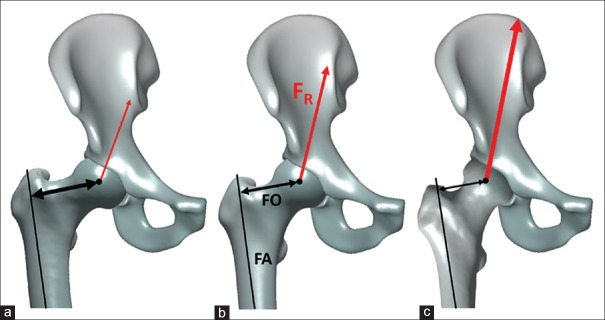
Figure 3.
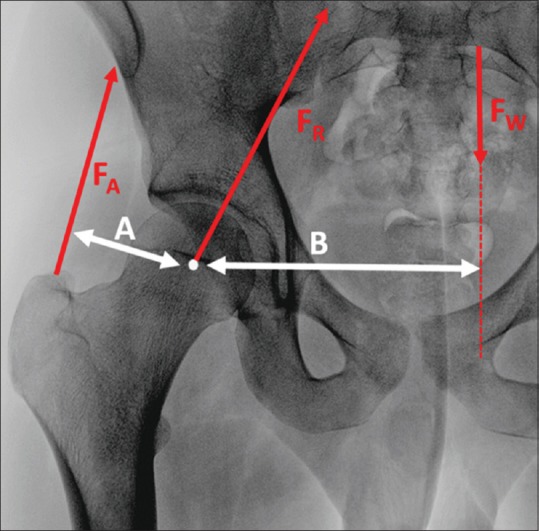
The static biomechanical model of unipodal stance during gait. The body weight vector (Fw), running perpendicular to the ground and originating from the center of mass, is counter balanced by the abductor force (FA). The magnitude of the bodyweight equals the bodyweight minus that of the weightbearing leg. The abductor force pulls along the trajectory of the gluteus medius/minimus muscle fibers. The bodyweight lever arm (B) is the perpendicular distance between the center of hip rotation and the bodyweight vector. As the center of mass moves laterally and the bodyweight increases, the abductor force will need to increase. The abductor lever arm (A) is the perpendicular distance between the center of hip rotation and the abductor force vector. If the abductor lever arm morphologically increases, the abductor force needed to counterbalance a given load decreases
Pauwels performed extensive research on the biomechanical impact of a varus and valgus configuration of the proximal femur. He acknowledged the influence of the neck-shaft angle on the reaction force of the hip and thereby the magnitude of stress on the femoral head. The theoretical reaction force is up to 25% lower in coxa vara compared with the average hip, whereas in coxa valga, it is 25% higher. The change in magnitude of the reaction force is caused by the change in the length of the abductor lever arm. As the neck-shaft angle increases, the abductor lever arm decreases, thereby requiring a higher abductor force to balance the BW.17
The Femoral Stem
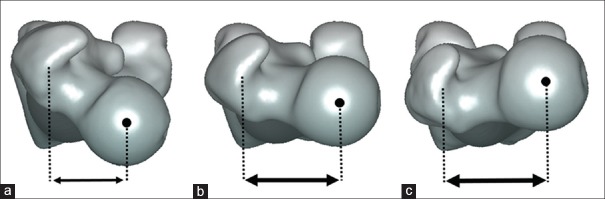
By the early 1990s, these static calculations led to a renewed interest in the restoration of femoral offset in THA.18,19 The femoral offset is the perpendicular distance from the center of rotation of the femoral head to the long axis of the femur.19 This two-dimensional (2D) radiographical measurement of a 3D structure varies depending on the rotation of the hip and thus requires X-rays to be taken in 15°–20° of internal rotation of the leg to expose the full length of the femoral neck on the anteroposterior views.20,21 The average value is 44 mm and increases with both the femoral size and decreasing neck-shaft angle [Figure 4].19,22 The average neck-shaft angle in Caucasians is approximately 130°, with males tending more toward varus.23,24 Besides the impact of the neck-shaft angle in the coronal plane, the amount of femoral version in the axial plane also influences the femoral offset.19,25 An increase in femoral neck anteversion, compared with the average femoral version of 9° in Caucasians,26 results in posterior displacement of the greater trochanter and decreases the abductor lever arm.27 [Figure 5] The decrease in “functional” offset has been shown to increase the hip joint reaction force.28 A large femoral offset is a reflection of a long abductor lever arm, which results in lower hip joint reaction forces. The theoretical advantage of a large femoral offset has been found to result in significantly decreased polyethylene wear in THA.29,30 Sakalkale et al.30 performed staged bilateral hip replacements in 17 patients with identical implants except for the femoral component being a lateral offset type in one hip compared with a standard offset in the other hip. At a mean followup of >5 years, the offset difference of 7 mm resulted in a wear rate of 0.21 mm/year in the standard offset THA compared to 0.10 mm/year in the lateral offset THA.
Figure 4.
Impact of neck-shaft angle on the femoral offset and hip joint reaction force. An increased neck-shaft angle results in a decrease in femoral offset and increase in hip joint reaction force. (a) Varus hip configuration of 115°, (b) mean Caucasian hip configuration with neck-shaft angle of 130°, (c) valgus hip configuration of 142°. FA: long axis of the femur, FO: femoral offset, FR: hip joint reaction force
Figure 5.
Impact of femoral version on the “functional” femoral offset. As the femoral anteversion increases, the femoral offset decreases resulting in higher hip joint reaction forces. (a) 35° of femoral anteversion, (b) 10° of physiological anteversion, (c) 10° of retroversion
Furthermore, restoring femoral offset during THA surgery is important because it controls the tension of the soft tissues, improves the overall functional outcome and abductor muscle strength.19,31,32 On the other hand, excessive femoral offset can lead to increased micromotion at the implant–bone interface,33 overloading of the femoral implant,34 and can be a cause for pain in the abductor complex and greater trochanteric region.35 Restoring a high native femoral offset, unfortunately, has the downside of increasing the varus and the rotational torque. Cantin et al.36 compared the fixation and survival of 280 lateralized stems to 527 standard Corail® cementless stems over an average of 2-year followup. In both groups, the choice for a lateralized or classic stem was based on restoring the native femoral offset and as such there was no significant difference in femoral offset change pre- and postoperatively between both groups. In the lateralized stem group, however, five cases of symptomatic aseptic loosening were diagnosed compared with none in the standard stem group.
The Acetabular Component
Implantation of the acetabular component of a THA is challenged by finding the optimal position as well as rotation of the cup.37 Failure to do so risks poor hip function,38,39 dislocation,40,41 squeaking of ceramic-on-ceramic components,42,43 and edge loading resulting in increased wear.29,44,45
The position of the cup is described in relation to the pelvis. The mediolateral implantation can be objectified by means of the acetabular offset: the shortest distance between the acetabular center of rotation and a perpendicular line to the interteardrop line, drawn along the projection of the most distal part of the teardrop.46,47 The sum of the acetabular and femoral offset is equal to the combined offset. Ideally, the combined offset is restored in THA surgery to maintain tension of the abductor muscle complex. Overtensioning can lead to iliotibial band friction and trochanteric pain, whereas undertensioning risks instability of the replaced hip.19,32,48 When the center of rotation of the replaced hip is lateralized compared with the center of rotation of the native hip, the femoral offset needs to decrease which in turn results in higher joint reaction forces and ultimately more wear.30 Medializing the acetabular cup on the other hand will allow for a larger femoral offset, less joint loading, and therefore reduced wear.30,49 This strategy can be useful in case of gluteal muscle insufficiency as well as to allow for a horizontal cup placement while maintaining sufficient bone coverage.45,50 Moving the cup superiorly has been shown to increase joint loading by 0.1% for every millimeter of superior displacement of the hip center of rotation, which is however seven times lower than the 0.7% increase/mm when lateralizing the center of rotation of the hip joint.51
In his biomechanical reference work, Pauwels also studied the importance of sufficient acetabular coverage and described the inversely proportional relationship between a decrease in weightbearing area and increase in joint pressure in dysplastic hips.17 Recent studies on contact stress distribution in the native hip joint confirmed the drastic increase of contact pressure in native hip joints with reduced anterior and especially reduced lateral coverage.52,53 By means of a mathematical model of hip joint contact stresses, Daniel et al.53 showed that the dysplastic hip joints (center-edge angle of 13°) suffer from an increase of over 100% in contact pressure compared with normal hip joints during staircase climbing and level walking. Hip joints with an increased acetabular anteversion (42°) exhibited 70%–115% higher peak joint stresses during climbing downstairs compared with hip joints with a low acetabular anteversion (7°).
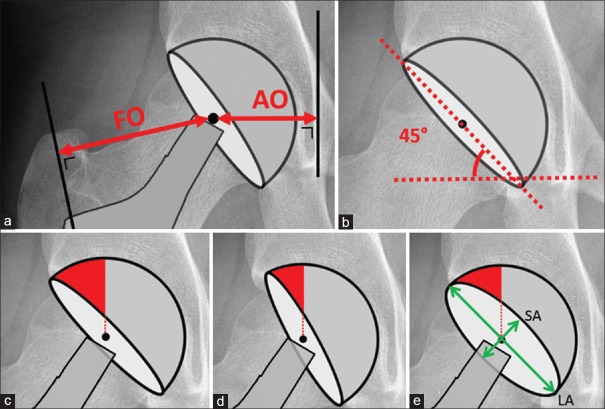
Acetabular component orientation in THA directly impacts on the risk of stem-cup impingement54,55,56 and dislocation.40,57 In the long term, the amount of weightbearing area mainly influences the wear rate.29,44,45,57,58,59 Radiological cup inclination is measured on an anteroposterior pelvic radiograph parallel to the anterior pelvic plane, i.e., a plane defined by both anterosuperior iliac spines and symphysis pubis.60,61 The cup abduction angle represents radiological cup inclination and is defined as the angle between the inter teardrop line and the major axis of the cup projection.60 [Figure 6] Radiographic cup anteversion is defined as the angle between the acetabular axis and the coronal plane and can be calculated with the proportion between the short and long axis on the anteroposterior pelvic view (anteversion = asin [short axis/long axis] × 180/π).60,61 When the abduction angle decreases, the acetabular component is more horizontal resulting in an increased anterosuperior weightbearing area but decreased posteroinferior coverage.62 Increasing the cup anteversion will decrease the anterosuperior weightbearing area and increase the posteroinferior coverage.62 The anterosuperior weightbearing area should be sufficiently large to distribute the joint loading. A sufficient posteroinferior coverage is necessary to stabilize the joint, especially during flexion, internal rotation, and adduction activities. Finally, femoral anteversion also plays an important role in stability because increasing the anteversion will allow for more internal rotation but at the price of earlier impingement of the posterior neck with the posterior acetabular rim during external rotation. An excess of femoral anteversion can therefore lead to an anterior dislocation. Femoral anteversion of the stem will be mainly determined by the shape of the proximal femur in uncemented stems, but can be altered by 10°–20° in cemented stems.63 To allow for a maximal impingement-free range of motion, the combined anteversion of the femoral stem and acetabular cup for a given cup inclination has to be evaluated.54,63,64,65 Overall, 40° ± 10° of cup abduction angle is advised together with a combined anteversion of approximately 40°.66,67 The required cup anteversion during surgery can be calculated as follows: cup anteversion = 40 − 0.7 × stem anteversion.55,64,68
Figure 6.
(a) Measurement of the AO and FO. (b) Cup abduction angle measured as the angle between the inter teardrop line and the major axis of the cup projection.60 (c) Anterosuperior weightbearing area in a cup with 45° of abduction and 15° anteversion, (d) reduced anterosuperior weightbearing area when cup is placed in 60° of abduction, (e) reduced anterosuperior weightbearing area with increased cup anteversion compared to image (c) of the same figure, green arrows representing SA and LA of the cup. Anteversion angle = asin (SA/LA) *180/pi. AO: Acetabular offset, FO: Femoral offset, SA: Short axis, LA: Long axis
Dynamic Biomechanics
From the late 1960 onward, the study of biomechanics has moved toward dynamic analysis, thanks to advancements made in sensors, processors, and personal computers. In 1966, the measurement of the first in vivo hip loading was pioneered by Rydell69 who implanted an instrumented hip prosthesis in two patients. He managed to measure hip force data at a few days and at 6 months postoperatively. In the following years, English and Kilvington70 and later on Davey et al.71 performed a similar procedure in three more patients. However, their experiments were all hampered by the limited battery life of the instrumented implants, required for radiofrequency transmission of the loading data. It was Georg Bergmann72 who reinvented the instrumented hip prosthesis in 1988 by eliminating the need for a battery. He equipped the implant with an internal telemetric sensor circuit powered inductively by an extra corporeal electromagnetic field.73 Four patients who consented to having this instrumented hip implant and mechanical loading being transferred data during diverse activities of daily life were observed in a gait laboratory more than a year postoperatively.74 It was shown that the average patient loads his/her hip joint with 238% BW when walking and with slightly less when standing on one leg. Climbing upstairs results in a peak joint reaction force of 251% BW and 260% BW when going downstairs. Inward torsion of the implant in the horizontal plane is of importance for the stem fixation.75 Peak torsional implant moment is highest during stair climbing, which is 23% higher than normal level walking and 78% higher during squatting.
By quantifying in vivo hip joint reaction forces and moments during different activities of daily living, Bergmann established a reference library. The Orthoload database76 was made publicly available and has ever since been considered as the gold standard for validating the computational estimation of joint loading by means of musculoskeletal models. Musculoskeletal models had been in use around previously, but it was not until the early 2000s, before comprehensive full-body musculoskeletal modeling packages such as AnyBody77 and OpenSim78 were developed. Again, the exponential progress in computational power made the development of these complex models possible. At their core, these software packages simulate rigid bodies representing the bones that are connected by mechanical joints. Muscles functioning as actuators provide the joint torque necessary to accelerate and as such move the body. The system can solve the associated equilibrium equations with an inverse dynamic approach: the external forces and motions are known and the internal forces have to be computed.77 Validated musculoskeletal hip models79,80 have been developed and allow for evaluation of activities of daily life, sports, or occupational activities as well as surgical strategies81 without the need for expensive and invasive in vivo experiments.
Next Generation
Previously, research was aimed at unraveling the average biomechanics of the hip joint and its implants. However, the trend has changed in the past 10 years and the demand for individual evaluation of joint biomechanics has dramatically increased. Recently a subject-specific scalable lower extremity model (TLEM 2.0) has been developed that accounts for individual changes in muscle lever arms and as such allows a more accurate estimation of individual hip joint reaction forces.82 Combined with finite element contact analysis, this enables the prediction of variations in strain through cartilage layers and peak joint pressures based on bony shape and dynamic use of the joint.83 Currently, the biomechanical evaluation of individual cases is still quite laborious. However, the exhilarating potential of dimension reduction algorithms will provide almost instantaneous prediction of the personalized loading of a hip implant in the nearby future.84
Conclusion
The study of biomechanics in THA started out with unraveling the general biomechanics of the hip joint and the impact of joint replacement. Over the years, the mathematical insights have proven to be indispensable in both design and surgical techniques. The nearby future holds promising answers in individualized evaluation of total hip surgery which will hopefully translate into improved functional restoration and longevity in THA.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Murphy LB, Helmick CG, Schwartz TA, Renner JB, Tudor G, Koch GG, et al. One in four people may develop symptomatic hip osteoarthritis in his or her lifetime. Osteoarthritis Cartilage. 2010;18:1372–9. doi: 10.1016/j.joca.2010.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Learmonth ID, Young C, Rorabeck C. The operation of the century: Total hip replacement. Lancet. 2007;370:1508–19. doi: 10.1016/S0140-6736(07)60457-7. [DOI] [PubMed] [Google Scholar]
- 3.Wiles P. The surgery of the osteoarthritic hip. Br J Surg. 1958;45:488–97. doi: 10.1002/bjs.18004519315. [DOI] [PubMed] [Google Scholar]
- 4.McKee GK, Watson-Farrar J. Replacement of arthritic hips by the McKee-Farrar prosthesis. J Bone Joint Surg Br. 1966;48:245–59. [PubMed] [Google Scholar]
- 5.13th Annual Report: National Joint Registry for England, Wales, Northern Ireland and the Isle of Man. National Joint Registry. 2016 [Google Scholar]
- 6.Callaghan JJ, Bracha P, Liu SS, Piyaworakhun S, Goetz DD, Johnston RC. Survivorship of a Charnley total hip arthroplasty. A concise followup, at a minimum of thirty-five years, of previous reports. J Bone Joint Surg Am. 2009;91:2617–21. doi: 10.2106/JBJS.H.01201. [DOI] [PubMed] [Google Scholar]
- 7.Wolff J. Ueber die Innere Architectur der Knochen. Virchows Arch. 1870;50:389. [Google Scholar]
- 8.Skedros JG, Brand RA. Biographical sketch: Georg Hermann von Meyer (1815-1892) Clin Orthop Relat Res. 2011;469:3072–6. doi: 10.1007/s11999-011-2040-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brodner W, Bitzan P, Lomoschitz F, Krepler P, Jankovsky R, Lehr S, et al. Changes in bone mineral density in the proximal femur after cementless total hip arthroplasty. A five-year longitudinal study. J Bone Joint Surg Br. 2004;86:20–6. [PubMed] [Google Scholar]
- 10.Glassman AH, Crowninshield RD, Schenck R, Herberts P. A low stiffness composite biologically fixed prosthesis. Clin Orthop Relat Res. 2001;393:128–36. doi: 10.1097/00003086-200112000-00015. [DOI] [PubMed] [Google Scholar]
- 11.Kärrholm J, Anderberg C, Snorrason F, Thanner J, Langeland N, Malchau H, et al. Evaluation of a femoral stem with reduced stiffness. A randomized study with use of radiostereometry and bone densitometry. J Bone Joint Surg Am. 2002;84-A:1651–8. [PubMed] [Google Scholar]
- 12.Tanzer M, Kantor S, Rosenthall L, Bobyn JD. Femoral remodeling after porous-coated total hip arthroplasty with and without hydroxyapatite-tricalcium phosphate coating: A prospective randomized trial. J Arthroplasty. 2001;16:552–8. doi: 10.1054/arth.2001.23721. [DOI] [PubMed] [Google Scholar]
- 13.Glassman AH, Bobyn JD, Tanzer M. New femoral designs: Do they influence stress shielding? Clin Orthop Relat Res. 2006;453:64–74. doi: 10.1097/01.blo.0000246541.41951.20. [DOI] [PubMed] [Google Scholar]
- 14.Bugbee WD, Culpepper WJ, 2nd, Engh CA, Jr, Engh CA., Sr Long term clinical consequences of stress-shielding after total hip arthroplasty without cement. J Bone Joint Surg Am. 1997;79:1007–12. doi: 10.2106/00004623-199707000-00006. [DOI] [PubMed] [Google Scholar]
- 15.Engh CA, Jr, Young AM, Engh CA, Sr, Hopper RH., Jr Clinical consequences of stress shielding after porous-coated total hip arthroplasty. Clin Orthop Relat Res. 2003;417:157–63. doi: 10.1097/01.blo.0000096825.67494.e3. [DOI] [PubMed] [Google Scholar]
- 16.Koch JC. The laws of bone architecture. Am J Anat. 1917;21:177–298. [Google Scholar]
- 17.Pauwels F. Biomechanics of the Normal and Diseased Hip: Theoretical Foundation, Technique, and Results of Treatment: An Atlas. Berlin, Heidelberg, New York: Springer; 1976. [Google Scholar]
- 18.Lindgren JU, Rysavy J. Restoration of femoral offset during hip replacement. A radiographic cadaver study. Acta Orthop Scand. 1992;63:407–10. doi: 10.3109/17453679209154755. [DOI] [PubMed] [Google Scholar]
- 19.Charles MN, Bourne RB, Davey JR, Greenwald AS, Morrey BF, Rorabeck CH. Soft-tissue balancing of the hip – The role of femoral offset restoration. J Bone Joint Surg Am. 2004;86A:1078–88. [PubMed] [Google Scholar]
- 20.Della Valle AG, Padgett DE, Salvati EA. Preoperative planning for primary total hip arthroplasty. J Am Acad Orthop Surg. 2005;13:455–62. doi: 10.5435/00124635-200511000-00005. [DOI] [PubMed] [Google Scholar]
- 21.Schmalzried TP. Preoperative templating and biomechanics in total hip arthroplasty. Orthopedics. 2005;28(8 Suppl):s849–51. doi: 10.3928/0147-7447-20050802-09. [DOI] [PubMed] [Google Scholar]
- 22.Davey JR, editor. Presented as an instructional course lecture at the Annual Meeting of the American Academy of Orthopaedic Surgeons. New Orleans, LA: 2003. Implant Issues in using High Offset Femoral Stems. [Google Scholar]
- 23.Boese CK, Jostmeier J, Oppermann J, Dargel J, Chang DH, Eysel P, et al. The neck shaft angle: CT reference values of 800 adult hips. Skeletal Radiol. 2016;45:455–63. doi: 10.1007/s00256-015-2314-2. [DOI] [PubMed] [Google Scholar]
- 24.Van Houcke J, Yau WP, Yan CH, Huysse W, Dechamps H, Lau WH, et al. Prevalence of radiographic parameters predisposing to femoroacetabular impingement in young asymptomatic Chinese and white subjects. J Bone Joint Surg Am. 2015;97:310–7. doi: 10.2106/JBJS.M.01538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tohtz SW, Heller MO, Taylor WR, Perka C, Duda GN. On the biomechanics of the hip: Relevance of femoral anteversion for hip contact force and loading using a short-stemmed prostheses. Orthopade. 2008;37:923–9. doi: 10.1007/s00132-008-1318-1. [DOI] [PubMed] [Google Scholar]
- 26.Koerner JD, Patel NM, Yoon RS, Sirkin MS, Reilly MC, Liporace FA. Femoral version of the general population: Does “normal” vary by gender or ethnicity? J Orthop Trauma. 2013;27:308–11. doi: 10.1097/BOT.0b013e3182693fdd. [DOI] [PubMed] [Google Scholar]
- 27.Lecerf G, Fessy MH, Philippot R, Massin P, Giraud F, Flecher X, et al. Femoral offset: Anatomical concept, definition, assessment, implications for preoperative templating and hip arthroplasty. Orthop Traumatol Surg Res. 2009;95:210–9. doi: 10.1016/j.otsr.2009.03.010. [DOI] [PubMed] [Google Scholar]
- 28.Kleemann RU, Heller MO, Stoeckle U, Taylor WR, Duda GN. THA loading arising from increased femoral anteversion and offset may lead to critical cement stresses. J Orthop Res. 2003;21:767–74. doi: 10.1016/S0736-0266(03)00040-8. [DOI] [PubMed] [Google Scholar]
- 29.Little NJ, Busch CA, Gallagher JA, Rorabeck CH, Bourne RB. Acetabular polyethylene wear and acetabular inclination and femoral offset. Clin Orthop Relat Res. 2009;467:2895–900. doi: 10.1007/s11999-009-0845-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sakalkale DP, Sharkey PF, Eng K, Hozack WJ, Rothman RH. Effect of femoral component offset on polyethylene wear in total hip arthroplasty. Clin Orthop Relat Res. 2001;388:125–34. doi: 10.1097/00003086-200107000-00019. [DOI] [PubMed] [Google Scholar]
- 31.McGrory BJ, Morrey BF, Cahalan TD, An KN, Cabanela ME. Effect of femoral offset on range of motion and abductor muscle strength after total hip arthroplasty. J Bone Joint Surg Br. 1995;77:865–9. [PubMed] [Google Scholar]
- 32.Cassidy KA, Noticewala MS, Macaulay W, Lee JH, Geller JA. Effect of femoral offset on pain and function after total hip arthroplasty. J Arthroplasty. 2012;27:1863–9. doi: 10.1016/j.arth.2012.05.001. [DOI] [PubMed] [Google Scholar]
- 33.O’Connor DO, Davey JR, Zalenski E, Burke DW, Harris WH. Femoral Component Offset: Its Effect on Micromotion in Stance and Stair Climbing Loading. Transactions 35th Annual Meeting of the Orthopaedic Research Society. 1989;14:409. [Google Scholar]
- 34.Davey J, O’connor D, Burke DW. Femoral component offset: Its effect on micromotion strain in the cement, bone and prosthesis. Orthop Translat. 1989;13:566. [Google Scholar]
- 35.Blackley HR, Howell GE, Rorabeck CH. Planning and management of the difficult primary hip replacement: Preoperative planning and technical considerations. Instr Course Lect. 2000;49:3–11. [PubMed] [Google Scholar]
- 36.Cantin O, Viste A, Desmarchelier R, Besse JL, Fessy MH. Compared fixation and survival of 280 lateralised vs.527 standard cementless stems after two years (1-7) Orthop Traumatol Surg Res. 2015;101:775–80. doi: 10.1016/j.otsr.2015.08.002. [DOI] [PubMed] [Google Scholar]
- 37.Scheerlinck T. Cup positioning in total hip arthroplasty. Acta Orthop Belg. 2014;80:336–47. [PubMed] [Google Scholar]
- 38.Kiyama T, Naito M, Shitama H, Maeyama A. Effect of superior placement of the hip center on abductor muscle strength in total hip arthroplasty. J Arthroplasty. 2009;24:240–5. doi: 10.1016/j.arth.2008.08.012. [DOI] [PubMed] [Google Scholar]
- 39.Bonnin MP, Archbold PH, Basiglini L, Selmi TA, Beverland DE. Should the acetabular cup be medialised in total hip arthroplasty. Hip Int. 2011;21:428–35. doi: 10.5301/HIP.2011.8582. [DOI] [PubMed] [Google Scholar]
- 40.Biedermann R, Tonin A, Krismer M, Rachbauer F, Eibl G, Stöckl B. Reducing the risk of dislocation after total hip arthroplasty: The effect of orientation of the acetabular component. J Bone Joint Surg Br. 2005;87:762–9. doi: 10.1302/0301-620X.87B6.14745. [DOI] [PubMed] [Google Scholar]
- 41.Espehaug B, Havelin LI, Engesaeter LB, Langeland N, Vollset SE. Patient-related risk factors for early revision of total hip replacements. A population register-based case-control study of 674 revised hips. Acta Orthop Scand. 1997;68:207–15. doi: 10.3109/17453679708996686. [DOI] [PubMed] [Google Scholar]
- 42.Kiyama T, Kinsey TL, Mahoney OM. Can squeaking with ceramic-on-ceramic hip articulations in total hip arthroplasty be avoided? J Arthroplasty. 2013;28:1015–20. doi: 10.1016/j.arth.2012.10.014. [DOI] [PubMed] [Google Scholar]
- 43.Affatato S, Traina F, Mazzega-Fabbro C, Sergo V, Viceconti M. Is ceramic-on-ceramic squeaking phenomenon reproducible in vitro? A long term simulator study under severe conditions. J Biomed Mater Res B Appl Biomater. 2009;91:264–71. doi: 10.1002/jbm.b.31398. [DOI] [PubMed] [Google Scholar]
- 44.De Haan R, Pattyn C, Gill HS, Murray DW, Campbell PA, De Smet K. Correlation between inclination of the acetabular component and metal ion levels in metal-on-metal hip resurfacing replacement. J Bone Joint Surg Br. 2008;90:1291–7. doi: 10.1302/0301-620X.90B10.20533. [DOI] [PubMed] [Google Scholar]
- 45.Wan Z, Boutary M, Dorr LD. The influence of acetabular component position on wear in total hip arthroplasty. J Arthroplasty. 2008;23:51–6. doi: 10.1016/j.arth.2007.06.008. [DOI] [PubMed] [Google Scholar]
- 46.Scheerlinck T. Primary hip arthroplasty templating on standard radiographs. A stepwise approach. Acta Orthop Belg. 2010;76:432–42. [PubMed] [Google Scholar]
- 47.Bonnin MP, Archbold PH, Basiglini L, Fessy MH, Beverland DE. Do we medialise the hip centre of rotation in total hip arthroplasty? Influence of acetabular offset and surgical technique. Hip Int. 2012;22:371–8. doi: 10.5301/HIP.2012.9350. [DOI] [PubMed] [Google Scholar]
- 48.Robinson M, Bornstein L, Mennear B, Bostrom M, Nestor B, Padgett D, et al. Effect of restoration of combined offset on stability of large head THA. Hip Int. 2012;22:248–53. doi: 10.5301/HIP.2012.9283. [DOI] [PubMed] [Google Scholar]
- 49.Košak R, Kralj-Iglic V, Iglic A, Daniel M. Polyethylene wear is related to patient-specific contact stress in THA. Clin Orthop Relat Res. 2011;469:3415–22. doi: 10.1007/s11999-011-2078-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yoo JJ, Yoon HJ, Yoon PW, Lee YK, Kim HJ. Medial placement of the acetabular component in an alumina-on-alumina total hip arthroplasty: A comparative study with propensity score matching. Arch Orthop Trauma Surg. 2013;133:413–9. doi: 10.1007/s00402-012-1661-x. [DOI] [PubMed] [Google Scholar]
- 51.Bicanic G, Delimar D, Delimar M, Pecina M. Influence of the acetabular cup position on hip load during arthroplasty in hip dysplasia. Int Orthop. 2009;33:397–402. doi: 10.1007/s00264-008-0683-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Genda E, Konishi N, Hasegawa Y, Miura T. A computer simulation study of normal and abnormal hip joint contact pressure. Arch Orthop Trauma Surg. 1995;114:202–6. doi: 10.1007/BF00444263. [DOI] [PubMed] [Google Scholar]
- 53.Daniel M, Iglic A, Kralj-Iglic V. Hip contact stress during normal and staircase walking: The influence of acetabular anteversion angle and lateral coverage of the acetabulum. J Appl Biomech. 2008;24:88–93. doi: 10.1123/jab.24.1.88. [DOI] [PubMed] [Google Scholar]
- 54.D’Lima DD, Urquhart AG, Buehler KO, Walker RH, Colwell CW., Jr The effect of the orientation of the acetabular and femoral components on the range of motion of the hip at different head-neck ratios. J Bone Joint Surg Am. 2000;82:315–21. doi: 10.2106/00004623-200003000-00003. [DOI] [PubMed] [Google Scholar]
- 55.Hisatome T, Doi H. Theoretically optimum position of the prosthesis in total hip arthroplasty to fulfill the severe range of motion criteria due to neck impingement. J Orthop Sci. 2011;16:229–37. doi: 10.1007/s00776-011-0039-1. [DOI] [PubMed] [Google Scholar]
- 56.Malik A, Maheshwari A, Dorr LD. Impingement with total hip replacement. J Bone Joint Surg Am. 2007;89:1832–42. doi: 10.2106/JBJS.F.01313. [DOI] [PubMed] [Google Scholar]
- 57.Kennedy JG, Rogers WB, Soffe KE, Sullivan RJ, Griffen DG, Sheehan LJ. Effect of acetabular component orientation on recurrent dislocation, pelvic osteolysis, polyethylene wear, and component migration. J Arthroplasty. 1998;13:530–4. doi: 10.1016/s0883-5403(98)90052-3. [DOI] [PubMed] [Google Scholar]
- 58.Hart AJ, Ilo K, Underwood R, Cann P, Henckel J, Lewis A, et al. The relationship between the angle of version and rate of wear of retrieved metal-on-metal resurfacings: A prospective, CT-based study. J Bone Joint Surg Br. 2011;93:315–20. doi: 10.1302/0301-620X.93B3.25545. [DOI] [PubMed] [Google Scholar]
- 59.Hart AJ, Muirhead-Allwood S, Porter M, Matthies A, Ilo K, Maggiore P, et al. Which factors determine the wear rate of large-diameter metal-on-metal hip replacements? Multivariate analysis of two hundred and seventy-six components. J Bone Joint Surg Am. 2013;95:678–85. doi: 10.2106/JBJS.J.01447. [DOI] [PubMed] [Google Scholar]
- 60.Murray DW. The definition and measurement of acetabular orientation. J Bone Joint Surg Br. 1993;75:228–32. doi: 10.1302/0301-620X.75B2.8444942. [DOI] [PubMed] [Google Scholar]
- 61.Lu M, Zhou YX, Du H, Zhang J, Liu J. Reliability and validity of measuring acetabular component orientation by plain anteroposterior radiographs. Clin Orthop Relat Res. 2013;471:2987–94. doi: 10.1007/s11999-013-3021-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Brown TD, Callaghan JJ. Impingement in total hip replacement: Mechanisms and consequences. Curr Orthop. 2008;22:376–391. doi: 10.1016/j.cuor.2008.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Dorr LD, Malik A, Dastane M, Wan Z. Combined anteversion technique for total hip arthroplasty. Clin Orthop Relat Res. 2009;467:119–27. doi: 10.1007/s11999-008-0598-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Widmer KH, Zurfluh B. Compliant positioning of total hip components for optimal range of motion. J Orthop Res. 2004;22:815–21. doi: 10.1016/j.orthres.2003.11.001. [DOI] [PubMed] [Google Scholar]
- 65.Barsoum WK, Patterson RW, Higuera C, Klika AK, Krebs VE, Molloy R. A computer model of the position of the combined component in the prevention of impingement in total hip replacement. J Bone Joint Surg Br. 2007;89:839–45. doi: 10.1302/0301-620X.89B6.18644. [DOI] [PubMed] [Google Scholar]
- 66.Lewinnek GE, Lewis JL, Tarr R, Compere CL, Zimmerman JR. Dislocations after total hip-replacement arthroplasties. J Bone Joint Surg Am. 1978;60:217–20. [PubMed] [Google Scholar]
- 67.McCollum DE, Gray WJ. Dislocation after total hip arthroplasty. Causes and prevention. Clin Orthop Relat Res. 1990;261:159–70. [PubMed] [Google Scholar]
- 68.Yoshimine F. The safe-zones for combined cup and neck anteversions that fulfill the essential range of motion and their optimum combination in total hip replacements. J Biomech. 2006;39:1315–23. doi: 10.1016/j.jbiomech.2005.03.008. [DOI] [PubMed] [Google Scholar]
- 69.Rydell NW. Forces acting on the femoral head-prosthesis. A study on strain gauge supplied prostheses in living persons. Acta Orthop Scand. 1966;37(Suppl 88):1–132. doi: 10.3109/ort.1966.37.suppl-88.01. [DOI] [PubMed] [Google Scholar]
- 70.English TA, Kilvington M. In vivo records of hip loads using a femoral implant with telemetric output (a preliminary report) J Biomed Eng. 1979;1:111–5. doi: 10.1016/0141-5425(79)90066-9. [DOI] [PubMed] [Google Scholar]
- 71.Davy DT, Kotzar GM, Brown RH, Heiple KG, Goldberg VM, Heiple KG, Jr, et al. Telemetric force measurements across the hip after total arthroplasty. J Bone Joint Surg Am. 1988;70:45–50. [PubMed] [Google Scholar]
- 72.Graichen F, Bergmann G. A telemetric transmission system for in vivo measuring of hip joint force with instrument-implanted prostheses. Biomed Tech (Berl) 1988;33:305–12. [PubMed] [Google Scholar]
- 73.Graichen F, Bergmann G. Four-channel telemetry system for in vivo measurement of hip joint forces. J Biomed Eng. 1991;13:370–4. doi: 10.1016/0141-5425(91)90016-z. [DOI] [PubMed] [Google Scholar]
- 74.Bergmann G, Deuretzbacher G, Heller M, Graichen F, Rohlmann A, Strauss J, et al. Hip contact forces and gait patterns from routine activities. J Biomech. 2001;34:859–71. doi: 10.1016/s0021-9290(01)00040-9. [DOI] [PubMed] [Google Scholar]
- 75.Kassi JP, Heller MO, Stoeckle U, Perka C, Duda GN. Stair climbing is more critical than walking in pre-clinical assessment of primary stability in cementless THA in vitro. J Biomech. 2005;38:1143–54. doi: 10.1016/j.jbiomech.2004.05.023. [DOI] [PubMed] [Google Scholar]
- 76.Bergmann G. Charité Universitaetsmedizin. Berlin: Orthoload; 2008. [Last accessed on 2017 May 20]. Available from: http://www.orthoload.com . [Google Scholar]
- 77.Damsgaard M, Rasmussen J, Christensen ST, Surma E, de Zee M. Analysis of musculoskeletal systems in the AnyBody Modeling System. Simul Model Pract Theory. 2006;14:1100–11. [Google Scholar]
- 78.Delp SL, Anderson FC, Arnold AS, Loan P, Habib A, John CT, et al. OpenSim: Open-source software to create and analyze dynamic simulations of movement. IEEE Trans Biomed Eng. 2007;54:1940–50. doi: 10.1109/TBME.2007.901024. [DOI] [PubMed] [Google Scholar]
- 79.Manders C, New A, Rasmussen J. Validation of musculoskeletal gait simulation for use in investigation of total hip replacement. J Biomech. 2008;41:S488. [Google Scholar]
- 80.Li J, McWilliams AB, Jin Z, Fisher J, Stone MH, Redmond AC, et al. Unilateral total hip replacement patients with symptomatic leg length inequality have abnormal hip biomechanics during walking. Clin Biomech (Bristol, Avon) 2015;30:513–9. doi: 10.1016/j.clinbiomech.2015.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Weber T, Al-Munajjed AA, Verkerke GJ, Dendorfer S, Renkawitz T. Influence of minimally invasive total hip replacement on hip reaction forces and their orientations. J Orthop Res. 2014;32:1680–7. doi: 10.1002/jor.22710. [DOI] [PubMed] [Google Scholar]
- 82.Carbone V, Fluit R, Pellikaan P, van der Krogt MM, Janssen D, Damsgaard M, et al. TLEM 2.0 – A comprehensive musculoskeletal geometry dataset for subject-specific modeling of lower extremity. J Biomech. 2015;48:734–41. doi: 10.1016/j.jbiomech.2014.12.034. [DOI] [PubMed] [Google Scholar]
- 83.Ateshian GA, Henak CR, Weiss JA. Toward patient-specific articular contact mechanics. J Biomech. 2015;48:779–86. doi: 10.1016/j.jbiomech.2014.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Viceconti M, Hunter P, Hose R. Big data, big knowledge: Big data for personalized healthcare. IEEE J Biomed Health Inform. 2015;19:1209–15. doi: 10.1109/JBHI.2015.2406883. [DOI] [PubMed] [Google Scholar]