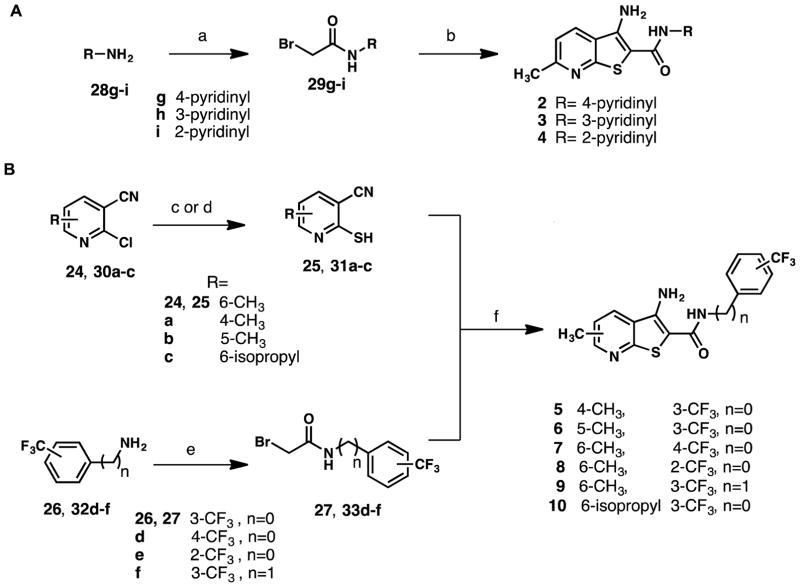
Scheme 2.
Synthetic routes to (A) pyridinyl analogs 2–4 (B) 5–10a
a Reagents and conditions: (a) bromoacetylbromide, DCM, rt, 3–12 h, 55%-95%; (b) 25, NaOEt, EtOH, reflux, 12 h, 32%-46%; (c) thiourea, n-BuOH, reflux, 3 h, quantitative; (d) Lawesson’s reagent, toluene, reflux, 4 h 30 min, 42%; (e) bromoacetyl bromide, DMAP, DCM, 0 °C to rt, 6–12 h, 62%-94%; (f) NaOEt, EtOH, reflux, 1 h, 23%-78%.