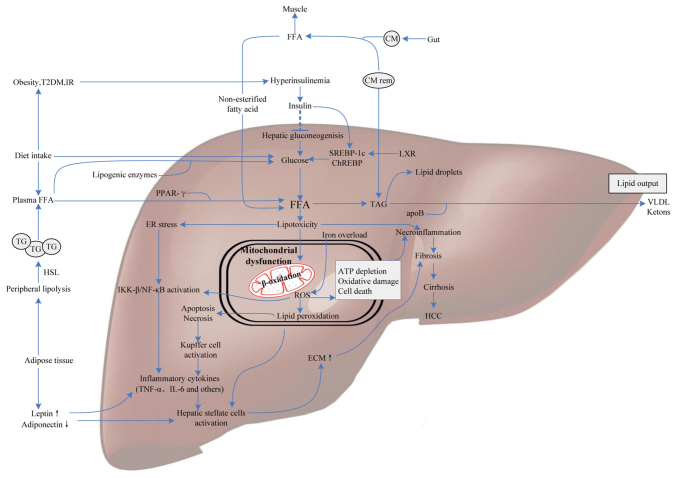
Figure 1.
Molecular mediators involved in NAFLD pathogenesis. FFAs derive from dietary fatty acids and lipolysis in the periphery, and then are delivered to the liver by CM via the portal vein. Meanwhile, individuals with obesity, IR or T2DM influence hepatic gluconeogenesis and transcriptional activity of SREBP-1c and ChREBP by increasing the generation of glucose, ultimately leading to intrahepatic FFA accumulation. Excess FFAs present lipotoxicity with the ability to impair hepatocytes triggering necroinflammation and mitochondrial dysfunction. Some FFAs in the liver are converted into TAG and then exported out of liver in the form of VLDL, others are oxidized in hepatic mitochondria, generating a mass of ROS. Additionally, iron overload generating hydroxyl radicals through the Fenton reaction also contributes to oxidative stress. ROS can cause oxidative damage, endoplasmic reticulum stress and apoptosis leading to necroinflammation via IKK-β activation, NF-κB activation and Kupffer cell activation pathways. Subsequently, the release of inflammatory cytokines, like TNF-α and IL-6, which give rise to hepatofibrogenesis, and finally progress to cirrhosis and HCC. Therefore, molecular mediators involved in glucose metabolism, fatty acid metabolism and oxidative stress may contribute to the progression of NAFLD. NAFLD, nonalcoholic fatty liver disease; FFAs, free fatty acids; IR, insulin resistance; CM, chylomicron; T2DM, type 2 diabetes; SREBP-1c, sterol regulatory element binding protein-1c; ChREBP, carbohydrate-responsive element-binding protein; TAG, triacylglycerol; VLDL, very low-density lipoprotein; ROS, reactive oxygen species; IKK-β, IkB kinase-β; NF-κB, nuclear factor-κB; HCC, hepatocellular carcinoma; PPAR, peroxisome proliferator-activated receptor; ApoB, apolipoprotein B; TG, triglyceride; ECM, extracellular matrix; TNF-α, tumor necrosis factor-α; LXR, liver X receptor; HSL, hormone-sensitive lipase.