Figure 1.
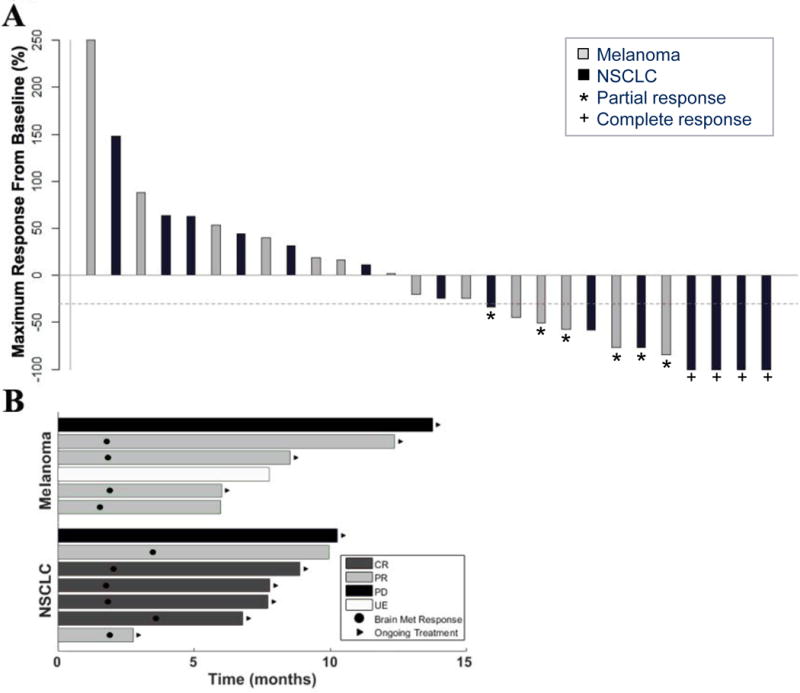
Characteristics of brain metastasis responses in patients with melanoma or NSCLC who received pembrolizumab. A. Best brain metastasis response by mRECIST in evaluable patients. Bars represent individual patients, with percent change in tumor burden in the brain from baseline to the best response demonstrated by the amplitude of the bars. Bar colors indicate patients with melanoma (gray) or NSCLC (black). The dashed line demonstrates the −30% cut-off that defines an objective response. Stars denote partial responses (PRs) and crosses denote complete responses (CRs). Two patients, one with melanoma and one with NSCLC, had PD despite >30% shrinkage of target lesions due to the development of new brain metastases and unequivocal progression of non-target lesions, respectively. The number of target (and total) untreated lesions among melanoma patients with a CNS response were 1 (1), 2 (6), 3 (5) and 3 (4) and among NSCLC patients 1 (3), 5 (58), 1 (2), 5 (50), 1 (3) and 1 (4). Eight patients were unevaluable in the brain, seven due to rapid systemic progression and one due to intralesional hemorrhage. B. Time to brain metastasis response and duration of therapy. Bars represent individual patients who achieved a brain metastasis response or remained on trial ≥ six months. Ten patients demonstrated an objective response in the brain by mRECIST (four with melanoma and six with NSCLC), and three additional patients remained on therapy ≥ six months due to clinical benefit despite having either disease progression or being unevaluable due to treatment of hemorrhagic lesions in the brain. Black circles indicate the time of first brain metastasis response, and black arrows indicate patients who continued on study treatment (regardless of response) at the time of data analysis.
