Abstract
Aims
To determine the extent to which older vs. younger adults with diabetes intensively control glycemia.
Methods
Participants were age ≥40 years who self-reported a physician diagnosis of diabetes in the 2009–2014 National Health and Nutrition Examination Surveys (N=1,554). Intensive glycemic control was defined as A1c<7.0% and taking insulin, sulfonylureas, or ≥2 glycemic medications. Logistic regression was used to determine the adjusted odds of intensive control in older (≥65 years) vs. younger adults (age 40–64 years).
Results
The prevalence of intensive control was greater for older (33.4%) vs. younger (21.3%) adults (p<0.001). In logistic regression, intensive control was significantly higher in older vs. younger adults after fully adjusting for sociodemographics, diabetes duration, comorbidities, disability, use of multiple medications, and depression (OR=1.72, 1.09–2.69). The multivariable adjusted prevalence of intensive control was 40% higher in adults ≥75 years (35.6%) compared to adults 40–49 years (21.7%).
Conclusions
Older adults are being treated more aggressively than younger adults to achieve A1c<7.0% despite the presence of comorbidities, duration of diabetes, disability, and depression. Glycemic guidelines for individualized therapy are not being widely followed.
Keywords: Diabetes, Treatment by age, NHANES
1. Introduction
An estimated 28.9 million U.S. adults have diabetes, yielding a large public health burden of morbidity, mortality and economic costs due mainly to diabetes-related complications1. The Diabetes Control and Complications Trial (DCCT) and the United Kingdom Prospective Diabetes Study (UKPDS) established that intensive glycemic control with a reduction in A1c levels to an average of 7.0% significantly reduced microvascular disease in persons with type 1 and type 2 diabetes2,3. Clinical practice guidelines developed on the basis of these findings recommended an A1c<7.0% to decrease the risk of diabetes complications. After the trials ended, significant long term reductions in cardiovascular disease (CVD) in those randomized to intensive treatment emerged during participant follow-up4. However, three subsequent studies, in older adults with longer duration type 2 diabetes and CVD or risk factors for CVD, found that more intensive therapy targeting even lower A1c levels did not reduce CVD5–7. Moreover, the Action to Control Cardiovascular Risk in Diabetes (ACCORD) trial, which targeted A1c<6.0%, increased mortality compared to the standard (A1c<7.5%) treatment group6.
On the basis of these studies, the ADA revised their guidelines in 2008 to recommend an A1c goal of <7.0% for adults who can benefit the most from a reduction in A1c to prevent diabetes-related complications, such as those with longer life expectancy and little comorbidity, but less stringent goals (e.g., A1c <8.0%) for patients with a history of hypoglycemia, advanced complications, several comorbid conditions, and shorter life expectancy8. Similarly, the American Geriatrics Society (AGS) first recommended in 2003 that individualized therapy take into account diabetes severity and life expectancy and recommended less stringent targets (e.g., A1c <8.0%) when the risks of intensive glycemic control outweighed the benefits9.
A previous study using national data from 2001–2010 found that among older adults with A1c<7.0% and significant health problems, 60% were treated with insulin or sulfonylureas; these results indicate possible overtreatment10. However, the practice patterns in younger adults with longer life expectancy, versus older adults, are relatively unknown. We used data from the National Health and Nutrition Examination Survey to determine the extent to which younger versus older adults are being treated more intensively to lower A1c levels while accounting for factors related to treatment, including duration of disease, comorbidities, disability, use of prescription medications, and depression.
2. Subjects, Materials, and Methods
2.1 Study Design and Participants
The National Health and Nutrition Examination Survey (NHANES) is a stratified multistage probability cluster survey conducted in the non-institutionalized civilian U.S. population11. Participants are interviewed in their home for demographic and health information and are then scheduled to visit a mobile examination center for physical examinations and laboratory measures12,13. Written informed consent was obtained from all participants and was approved by the National Center for Health Statistics Institutional Review Board. Our analyses included adults age ≥40 years who answered “yes” when asked whether a physician or other health care professional ever told them they had diabetes (N=1,554). We excluded adults with probable type 1 diabetes defined as having a diagnosis at age <30 years, starting insulin treatment within one year of diagnosis, and currently taking insulin (n=21). Participants self-reported age, race/ethnicity, education, health insurance status, smoking status, duration of diabetes, and use of insulin.
2.2 Health Status
Self-reported comorbidities included a history of cancer (excluding skin cancer, except Melanoma), lung disease (asthma, bronchitis, or emphysema), cardiovascular disease (stroke, congestive heart failure, coronary heart disease, angina, or heart attack). Chronic kidney disease (CKD) was determined using the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation which estimates glomerular filtration rate (eGFR) from serum creatinine based on age, sex, and race and defined as eGFR<60mL/min per 1.73m2 14. Participants with ≥1 of these conditions were considered to have comorbidities.
Disability was defined as having mobility disability, work disability, or general pain. Participants who reported needing special equipment to walk or much difficulty/inability to do any of the following activities were considered to have a mobility disability: (1) walking a quarter mile, (2) walking up ten steps, (3) stooping, crouching, or kneeling, (4) walking between rooms, (5) standing up from an armless chair, (6) getting in and out of bed. A work disability was determined if participants responded “yes” when asked if limitations kept them from working. Participants who reported ≥3 days in the past month where pain made it hard for usual activities were considered to have disability related to general pain.
Participants self-reported use of prescription medications in the past 30 days and were asked to show the interviewer their medication containers. Use of ≥6 prescription medications was considered multiple medication use.
The Patient Health Questionnaire (PHQ9) was used to determine depression15. Participants answered 9 questionnaire items with “not at all” (value of 0), “several days” (value of 1), “more than half the days” (value of 2), or “nearly every day” (value of 3) in the past two weeks.” Symptoms included (1) little interest in doing things, (2) feeling down, depressed, or hopeless, (3) trouble sleeping or sleeping too much, (4) feeling tired or having little energy, (5) poor appetite or overeating, (6) feeling bad about yourself, (7) trouble concentrating on things, (8) moving or speaking slowly or too fast, (9) would be better off dead. Depression was defined as having a PHQ9 score ≥10.
2.3 Intensive Control of Diabetes
Intensive control of diabetes was defined as A1c<7.0% and use of sulfonylureas, insulin, or ≥2 glycemic medications. The DCCT used insulin and the UKPDS used insulin and sulfonylureas as intensive therapy to achieve A1c control2,3 and taking 2 or more glycemic medications indicates that diabetes cannot be controlled with lifestyle or first line medications. This definition of intensive control is not based on A1c alone but in the context of pharmacological therapy (and potential detriment) required to achieve near normal A1c. The comparison in older and younger adults is the use of this potentially harmful pharmacological therapy to achieve A1c<7.0%. Hemoglobin A1c was measured in all adults from a standard blood draw and standardized to the Diabetes Control and Complications Trial method16. A1c was measured with the A1c G7 HPLC Glycohemoglobin Analyzer (Tosoh Medics, Inc., San Francisco, CA) which had a coefficient of variation of 0.7–1.5%. Sulfonylurea use was determined during the prescription medication interview; insulin use was self-reported and adjudicated with the prescription medication interview.
2.4 Statistical Analysis
Distributions of demographic factors, health insurance, smoking status, and duration of diabetes and prevalences of comorbidities, disability, use of multiple medications, and depression were determined by age (40–64 years vs. ≥65 years). Analysis of variance (F-test) was used to determine differences in the distribution of participant characteristics. Prevalence of intensive control of diabetes was determined by age and by demographics, presence of comorbidities, disability, use of multiple medications, and depression. Differences in means were tested by two-tailed large sample z-tests. Logistic regression (odds ratio [OR], 95% confidence interval [CI]) was used to determine the odds of older vs. younger adults having intensive control of diabetes. Models were (1) unadjusted, (2) adjusted for sex and race/ethnicity, (3) additionally adjusted for health insurance and education, (4) additionally adjusted for duration of diabetes, (5) additionally adjusted for smoking, (6) additionally adjusted for comorbidities, disability, and use of multiple medications, (9) and additionally adjusted for depression. Predictive margins regression was used to determine the prevalence of intensive control by smaller age groups (40–49, 50–64, 65–74, ≥75 years); models were adjusted for covariates in the same order as for the logistic regression analysis. These regression techniques account for differences in participant characteristics (e.g. comorbidities, duration of diabetes), by averaging these characteristics across all subgroups and creating age groups with participants who have similar levels of comorbidities, duration of diabetes, disability, medication use, and depression. All statistical analyses used sample weights and accounted for the cluster design using SUDAAN (SUDAAN User’s Manual, Release 9.2, 2008; Research Triangle Institute).
3. Results
3.1 Characteristics of the Study Population
Fifty-five percent (55.2%) were age 40–64 years (median age 54.4 years) and 44.8% were ≥65 years (median age 72.1 years); 48.8% were women with no difference by age group (Table 1). The majority was non-Hispanic white (61.4%), and most had a high school or more education (73.4%). Almost all adults with type 2 diabetes age ≥65 years had health insurance (98.1%) compared to 85.1% of those age 40–64 years (p<0.001). More adults age 40–64 years were current smokers compared to those age ≥65 years (p<0.001). Duration of diabetes ≥20 years was higher for adults age ≥65 years compared to 40–64 years (p<0.001). The prevalence of comorbidities, disability, and use of multiple medications was greater for those age ≥65 years vs. 40–64 years (p≤0.006 for all). More adults age 40–64 years vs. ≥65 years reported depression (p<0.001).
Table 1.
Characteristics of adults with type 2 diabetes age ≥40 years, NHANES 2009–2014
| Percent (standard error) | ||||
|---|---|---|---|---|
| Total (N=1,554) |
Age 40–64 Years (n=793) |
Age ≥65 Years (n=761) |
ANOVA p-value |
|
| Age | 100.0 | 55.2 (1.51) | 44.8 (1.51) | 0.005 |
| Sex, % women | 48.8 (1.67) | 49.2 (2.28) | 48.2 (2.61) | 0.778 |
| Race/Ethnicity | <0.001 | |||
| Non-Hispanic white | 61.4 (2.31) | 57.1 (2.87) | 66.8 (2.49) | |
| Non-Hispanic black | 15.8 (1.67) | 17.8 (2.07) | 13.3 (1.49) | |
| Mexican American | 8.6 (1.55) | 11.0 (1.88) | 5.6 (1.37) | |
| Other Hispanic | 5.0 (0.77) | 5.5 (0.93) | 4.4 (0.82) | |
| Non-Hispanic Other | 9.2 (1.27) | 8.6 (1.30) | 10.0 (1.67) | |
| Education, % high school graduate or higher | 73.4 (1.89) | 75.7 (2.14) | 70.6 (2.30) | 0.029 |
| Health Insurance, % yes | 90.9 (0.97) | 85.1 (1.67) | 98.1 (0.63) | <0.001 |
| Current Smoking | 14.0 (0.92) | 20.6 (1.52) | 5.8 (0.84) | <0.001 |
| Duration of Diabetes (years) | <0.001 | |||
| <5 | 27.9 (1.42) | 34.9 (2.05) | 19.3 (1.88) | |
| 5–9 | 23.0 (1.19) | 24.6 (1.91) | 20.9 (1.44) | |
| 10–19 | 31.2 (1.91) | 30.0 (2.48) | 32.7 (2.06) | |
| ≥20 | 17.9 (1.18) | 10.5 (1.40) | 27.1 (1.88) | |
| Comorbidities, ≥1* | 60.4 (1.71) | 47.5 (2.20) | 76.7 (1.73) | <0.001 |
| Disability† | 67.4 (2.25) | 63.2 (2.95) | 72.3 (2.48) | 0.006 |
| Multiple Medication Use, % ≥6 medications | 56.6 (1.97) | 49.0 (2.65) | 66.0 (2.04) | <0.001 |
| Depression‡ | 12.4 (1.18) | 16.2 (1.72) | 7.8 (1.34) | <0.001 |
Comorbidities include cancer (excluding skin cancer except Melanoma), lung disease (asthma, bronchitis, or emphysema), cardiovascular disease (stroke or CVD), and chronic kidney disease
Disability includes work disability, mobility disability, or pain ≥3 days in past month that makes daily activities difficult
Depression defined as a PHQ9 score ≥10
3.2 Prevalence of Intensive Control of Diabetes
The prevalence of intensive control was higher for adults age ≥65 years vs. age 40–64 years (p<0.001) (Table 2). This relationship by age was consistent for women, all race/ethnicities, and education levels; for those with health insurance; for never or former smokers; and for those with duration of diabetes of 5–19 years (p<0.05 for all).
Table 2.
Prevalence of intensive glycemic control of type 2 diabetes among adults age ≥40 years, NHANES 2009–2014
| Percent (standard error) | ||||
|---|---|---|---|---|
| Total (N=1,554) |
Age 40–64 Years (n=793) |
Age ≥65 Years (n=761) |
p-value | |
| Age | ||||
| Total | 26.7 (1.59) | 21.3 (2.32) | 33.4 (2.18) | <0.001 |
| Sex | ||||
| Men | 27.7 (2.07) | 23.2 (3.02) | 33.1 (3.53) | 0.057 |
| Women | 25.7 (2.03) | 19.3 (2.64) | 33.7 (2.39) | <0.001 |
| Race/Ethnicity | ||||
| Non-Hispanic white | 29.1 (2.29) | 22.7 (3.52) | 35.8 (3.01) | 0.007 |
| Non-Hispanic black | 27.7 (2.52) | 23.9 (2.82) | 34.1 (4.28) | 0.042 |
| Mexican American | 16.5 (1.90) | 13.2 (2.49) | 24.7 (4.00) | 0.032 |
| Other Hispanic | 17.3 (3.23) | 12.4 (3.33) | 24.7 (4.92) | 0.034 |
| Education | ||||
| <HS | 31.6 (2.41) | 23.8 (3.46) | 39.5 (3.21) | 0.001 |
| ≥HS | 24.9 (1.97) | 20.5 (2.71) | 30.9 (2.84) | 0.011 |
| Health Insurance | ||||
| No | 13.5 (3.93) | 13.6 (4.20) | 12.7 (7.08) | 0.914 |
| Yes | 28.1 (1.73) | 22.6 (2.49) | 33.9 (2.30) | 0.001 |
| Smoking | ||||
| Current | 21.8 (3.62) | 20.0 (4.06) | 29.7 (7.81) | 0.267 |
| Never/former | 27.5 (1.78) | 21.6 (2.71) | 33.7 (2.38) | 0.002 |
| Duration of Diabetes (years) | ||||
| <5 | 26.0 (2.88) | 26.1 (4.01) | 25.8 (3.55) | 0.963 |
| 5–9 | 26.6 (3.23) | 18.9 (3.89) | 37.9 (5.76) | 0.010 |
| 10–19 | 23.6 (2.30) | 16.5 (2.60) | 31.8 (3.91) | 0.002 |
| ≥20 | 32.9 (3.52) | 23.1 (6.23) | 37.6 (4.05) | 0.054 |
| Comorbidities ≥1* | ||||
| No | 21.0 (2.23) | 17.9 (2.90) | 29.7 (4.85) | 0.067 |
| Yes | 30.7 (2.21) | 24.8 (3.41) | 35.3 (2.75) | 0.018 |
| Cancer | ||||
| No | 25.8 (1.79) | 21.1 (2.57) | 32.4 (2.29) | 0.002 |
| Yes | 32.4 (4.44) | 24.5 (8.93) | 37.7 (4.62) | 0.203 |
| Lung Disease | ||||
| No | 25.8 (2.00) | 20.9 (2.86) | 31.6 (2.37) | 0.004 |
| Yes | 29.2 (2.92) | 22.4 (4.03) | 38.4 (4.93) | 0.019 |
| Cardiovascular Disease | ||||
| No | 25.3 (1.71) | 21.1 (2.54) | 32.4 (2.97) | 0.013 |
| Yes | 30.3 (3.45) | 22.4 (4.48) | 35.1 (3.80) | 0.008 |
| Chronic Kidney Disease | ||||
| No | 24.0 (1.72) | 20.0 (2.28) | 31.7 (3.25) | 0.009 |
| Yes | 35.8 (3.32) | 31.5 (6.35) | 37.1 (3.65) | 0.426 |
| Disability† | ||||
| No | 26.7 (3.24) | 21.6 (4.81) | 34.6 (5.21) | 0.098 |
| Yes | 29.4 (1.80) | 21.7 (2.48) | 37.3 (2.52) | <0.001 |
| Work Disability | ||||
| No | 25.3 (2.08) | 20.8 (3.17) | 30.4 (2.79) | 0.032 |
| Yes | 30.7 (2.78) | 22.5 (2.25) | 43.1 (4.92) | <0.001 |
| Mobility Disability | ||||
| No | 25.5 (2.33) | 22.4 (3.28) | 30.3 (3.74) | 0.147 |
| Yes | 28.8 (2.25) | 18.6 (2.78) | 37.4 (3.14) | <0.001 |
| Pain, general | ||||
| No | 29.5 (2.73) | 22.0 (4.51) | 37.7 (3.96) | 0.019 |
| Yes | 32.2 (2.50) | 22.8 (4.02) | 43.7 (3.89) | 0.002 |
| Multiple Medication Use, ≥6 medications | ||||
| No | 20.4 (2.19) | 19.2 (2.99) | 22.7 (3.18) | 0.454 |
| Yes | 31.5 (2.27) | 23.4 (3.32) | 39.0 (3.18) | 0.001 |
| Number of Medications | ||||
| 1–3 | 16.1 (3.73) | 15.8 (4.71) | 17.0 (4.87) | 0.848 |
| 4–5 | 23.6 (2.51) | 22.1 (3.81) | 25.9 (3.90) | 0.533 |
| 6–9 | 29.1 (2.66) | 22.7 (3.79) | 34.8 (3.65) | 0.026 |
| ≥10 | 37.2 (4.46) | 24.9 (6.50) | 49.6 (4.83) | 0.002 |
| Depression (PHQ9 Score)‡ | ||||
| <10 | 26.9 (1.69) | 20.9 (2.67) | 33.5 (2.48) | 0.003 |
| ≥10 | 21.3 (2.84) | 18.2 (3.24) | 29.3 (6.64) | 0.171 |
Intensive control of diabetes is defined as A1c<7.0% and taking insulin or sulfonylurea or ≥2 glycemic medications
Comorbidities include cancer (excluding skin cancer except Melanoma), lung disease (asthma, bronchitis, or emphysema), cardiovascular disease (stroke or CVD), and chronic kidney disease
Disability includes work disability, mobility disability, or pain ≥3 days in past month that makes daily activities difficult
Depression defined as a PHQ9 score ≥10
The prevalence of intensive control of diabetes was higher among those age ≥65 years with comorbidities compared to those age 40–64 years with comorbidities (p=0.018). In addition, prevalence of intensive control was higher among adults age ≥65 years with disability (p<0.001), or using multiple medications (p=0.001), compared to their counterparts age 40–64 years.
3.3 Logistic Regression Analysis
Adults age ≥65 years were significantly more likely to use intensive control compared to those age 40–64 years after adjusting for demographics, duration of diabetes, smoking, comorbidities, disability, use of multiple medications, and depression (OR=1.72, 1.09–2.69) (Table 3). Thus, regardless of the differences in health status by age, these results indicate that older adults were more likely to have intensive glycemic control compared to younger adults. The adjusted association was slightly attenuated from the unadjusted model.
Table 3.
Odds ratio (95% confidence interval) for intensive glycemic control of type 2 diabetes, NHANES 2009–2014
| Model 1 | Model 2 | Model 3 | Model 4 | Model 5 | Model 6 | Model 7 | |
|---|---|---|---|---|---|---|---|
| Unadjusted | Adjusted for Sex, Race/Ethnicity |
Model 2 + Health Insurance, Education |
Model 3+ Duration of Diabetes |
Model 4+ Smoking |
Model 5+ Comorbidity, Disability, Multiple Medication Use* |
Model 6 + Depression† |
|
| Age (years) | |||||||
| 40–64 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| ≥65 | 1.86 (1.31, 2.63) | 1.81 (1.26, 2.58) | 1.64 (1.15, 2.33) | 1.61 (1.12, 2.27) | 1.59 (1.10, 2.32) | 1.77 (1.22, 2.56) | 1.72 (1.09, 2.69) |
Comorbidities include cancer (excluding skin cancer except Melanoma), lung disease (asthma, bronchitis, or emphysema), cardiovascular disease (stroke or CVD), and chronic kidney disease; Disability includes work disability, mobility disability, or pain ≥3 days in past month that makes daily activities difficult; Use of ≥6 prescription medications
Depression defined as a PHQ9 score ≥10
3.4 Adjusted Prevalence of Intensive Control of Diabetes
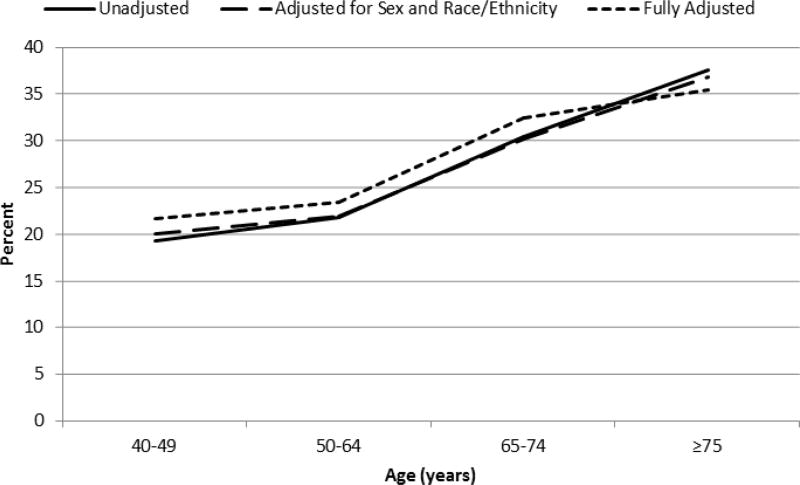
The prevalence of intensive control increased with increasing age; unadjusted estimates and estimates adjusted for sex and race/ethnicity were similar (Figure 1, Appendix Table A). The magnitude of intensive control was slightly attenuated for older adults in the fully adjusted model but still remained significantly higher compared to younger adults. In the fully adjusted model, the prevalence of intensive control was 21.7%, 23.5%, 32.5%, and 35.6% for those age 40–49, 50–64, 65–74, and ≥75 years, respectively.
Figure 1.
Prevalence of intensive glycemic control by age among adults with type 2 diabetes, NHANES 2009–2014. Fully adjusted model includes sex, race/ethnicity, health insurance, education, duration of diabetes, smoking, comorbidities, disability, multiple medication use, and depression.
4. Discussion
Nearly 26 percent of people age ≥65 years had diabetes in 2012, with a substantial increase in the number of older adults with diabetes expected in the coming decades1. There is considerable heterogeneity in this group. The older population with diabetes includes individuals newly diagnosed with diabetes and as well as those with longer durations of disease who may have established complications. While diabetes in older adults is associated with increased mortality and reduced functional status, the population also includes those with substantial life expectancy and without comorbid conditions. Thus, recommendations for glycemic targets are not age specific but rather depend on individualized assessments of the risks and benefits of treatment: balancing factors such as life expectancy of sufficient duration to realize reduction in microvascular complications with glycemic control and risk of hypoglycemia and other harms of glucose lowering medications.
While age may not be the major determinant of life expectancy for individuals, in the population as a whole, age does strongly correlate with life expectancy. Thus, it would be expected that population-wide the implementation of individualized goals for therapy, in which life expectancy is a major factor, would lead to less intensive control on average in older versus younger populations with diabetes. We found that, regardless of demographics, duration of diabetes, comorbidities, and other diabetes-related factors, older adults were more likely to have intensive glycemic control compared to their younger counterparts. Moreover, there was roughly a 40% increase in the adjusted prevalence of intensive glycemic control for adults age ≥75 years vs. age 40–49 years. Thus, despite treatment recommendations by the ADA and AGS which endorse less stringent goals for patients with shorter life expectancy and comorbidities,17,18 older adults are being treated more aggressively than younger adults.
Our results agree with a recent national study which found that adults age ≥65 years were achieving tight glycemic targets regardless of poor health status and that most of these patients were treated with insulin or sulfonylureas10. However, that study did not compare younger and older populations with diabetes and was unable to assess any response to the change in ADA Guidelines after the ACCORD results were released.
Recent data indicate that hypoglycemia has surpassed hyperglycemia as a cause of emergency room visits and hospitalizations19. About 25% of hospitalizations for adverse drug events in U.S. adults age ≥65 years are due to insulin or oral hypoglycemic agents20. Thus, diabetes agents were selected as one of the top three concerns in the recent National Action Plan for Adverse Drug Events21. The ADA, AGS, and the Department of Veterans Affairs/Department of Defense all recommend that risk of hypoglycemia be considered in recommending a target A1c goal8,9,22. Older adults may be particularly vulnerable to hypoglycemia23. After adjustment for duration of diabetes, rates of hypoglycemia increase sharply with advancing age24. Some individuals, particularly early in the course of diabetes, may achieve tight glucose control with low risk therapies such as lifestyle modifications or metformin. However, our definition of intensive control required use of sulfonylurea, insulin or at least two anti-diabetic agents. We found that despite their increased risk of hypoglycemia older adults were more likely to receive intensive glycemic control compared to younger adults, even after adjustment for duration of diabetes.
Several factors may contribute to more intensive treatment of older adults even after controlling for diabetes-related factors. First, older individuals may have more interaction with doctors due to multiple medical problems. Second, older adults generally have more time to take care of their health and/or are more concerned about their health than younger adults. Third, although our finding of more intensive control was not altered by adjustment for insurance status, Medicare coverage may lessen barriers to intensive control compared to insurance with more out of pocket costs. Finally, diabetes in older adults may be less complicated and easier to treat due to survival bias25.
One strength of our study is the use of nationally representative data allowing generalization to the U.S. adult non-institutionalized civilian population. The NHANES provides standardized measures of A1c to characterize, in part, intensive glycemic control. The NHANES included several covariates, including duration of diabetes, comorbidities, disability, prescription medication use, and depression, which allowed us to adjust our analysis for these confounding factors. One limitation is the reliance on self-reported medication use and the lack of information on medication adherence. In addition, information on the length of time participants were on intensive treatment to achieve A1c<7.0% was not available.
4.1 Conclusions
Guidelines for glycemic control call for individualized targets that weigh the risks and benefits of intensive therapy. While age is not a suitable basis for therapeutic decision-making in individual patients, in the population at large it would be expected to correlate with health status. Indeed, we found that older adults had more comorbidities, disability, and use of multiple medications than younger adults and, even after controlling for these factors, older adults were treated more aggressively than younger adults with agents that carry the risk of hypoglycemia. This suggests that the guidelines for individualized therapy are not being widely followed.
Acknowledgments
Funding Source: This work was financially supported by the National Institute of Diabetes and Digestive and Kidney Diseases (GS-10F-0381L).
None
Appendix
Table A.
Prevalence (standard error) of intensive glycemic control adjusted for covariates among adults with type 2 diabetes, NHANES 2009–2014
| Model 1 | Model 2 | Model 3 | Model 4 | Model 5 | Model 6 | Model 7 | |
|---|---|---|---|---|---|---|---|
| Unadjusted | Adjusted for Sex, Race/Ethnicity |
Model 2+ Health Insurance, Education |
Model 3+ Duration of Diabetes |
Model 4+ Smoking |
Model 5+ Comorbidities, Disability, Multiple Medication Use* |
Model 6 + Depression† |
|
| Age | |||||||
| 40–49 | 19.3 (3.46) | 20.1 (3.71) | 21.3 (3.84) | 21.3 (3.85) | 21.4 (3.91) | 20.1 (3.60) | 21.7 (3.89) |
| 50–64 | 21.9 (2.50) | 22.0 (2.52) | 22.6 (2.58) | 22.5 (2.55) | 22.8 (2.61) | 23.8 (3.04) | 23.5 (3.42) |
| 65–74 | 30.5 (2.88) | 30.3 (2.83) | 29.6 (2.67) | 29.8 (2.63) | 29.5 (2.63) | 33.0 (3.12) | 32.5 (3.24) |
| ≥75 | 37.7 (3.31) | 36.9 (3.30) | 35.0 (3.27) | 34.5 (3.37) | 34.1 (3.53) | 36.1 (3.99) | 35.6 (4.29) |
Comorbidities include cancer (excluding skin cancer except Melanoma), lung disease (asthma, bronchitis, or emphysema), cardiovascular disease (stroke or CVD), chronic kidney disease (CKD-EPI equation); Disability includes work disability, mobility disability, or pain ≥3 days in the past month that makes daily activities difficult; Use of ≥6 prescription medications
Depression defined as a PHQ9 score ≥10
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclaimer: The contents of this letter represent the author’s views and do not constitute an official position of the Department of Health and Human Services.
Conflicts of Interest: None
Contributor Information
Sarah Casagrande, Social & Scientific Systems, Inc., 8757 Georgia Ave., Silver Spring, MD, USA 20910, Phone: 301-628-3297; Fax: 301-628-3001, scasagrande@s-3.com.
Catherine C. Cowie, National Institute of Diabetes and Digestive and Kidney Diseases, 6707 Democracy Blvd, Bethesda, MD, USA 20892, cowie@nih.gov.
Judith E. Fradkin, National Institute of Diabetes and Digestive and Kidney Diseases, 6707 Democracy Blvd, Bethesda, MD, USA 20892, Judith.Fradkin@nih.hhs.gov.
References
- 1.Standards of Medical Care in Diabetes-2016: Summary of Revisions. Diabetes Care. 2016 Jan;39(Suppl 1):S4–5. doi: 10.2337/dc16-S003. [DOI] [PubMed] [Google Scholar]
- 2.The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. The Diabetes Control and Complications Trial Research Group. N Engl J Med. 1993 Sep 30;329(14):977–986. doi: 10.1056/NEJM199309303291401. [DOI] [PubMed] [Google Scholar]
- 3.Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). UK Prospective Diabetes Study (UKPDS) Group. Lancet. 1998 Sep 12;352(9131):837–853. [PubMed] [Google Scholar]
- 4.Holman RR, Paul SK, Bethel MA, Matthews DR, Neil HA. 10-year follow-up of intensive glucose control in type 2 diabetes. N Engl J Med. 2008 Oct 9;359(15):1577–1589. doi: 10.1056/NEJMoa0806470. [DOI] [PubMed] [Google Scholar]
- 5.Duckworth W, Abraira C, Moritz T, et al. Glucose control and vascular complications in veterans with type 2 diabetes. N Engl J Med. 2009 Jan 8;360(2):129–139. doi: 10.1056/NEJMoa0808431. [DOI] [PubMed] [Google Scholar]
- 6.Gerstein HC, Miller ME, Byington RP, et al. Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med. 2008 Jun 12;358(24):2545–2559. doi: 10.1056/NEJMoa0802743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Patel P, Macerollo A. Diabetes mellitus: diagnosis and screening. American family physician. 2010 Apr 1;81(7):863–870. [PubMed] [Google Scholar]
- 8.Standards of medical care in diabetes--2008. Diabetes Care. 2008 Jan;31(Suppl 1):12–54. doi: 10.2337/dc08-S012. [DOI] [PubMed] [Google Scholar]
- 9.Brown AF, Mangione CM, Saliba D, Sarkisian CA. Guidelines for improving the care of the older person with diabetes mellitus. Journal of the American Geriatrics Society. 2003 May;51(5 Suppl Guidelines):S265–280. doi: 10.1046/j.1532-5415.51.5s.1.x. [DOI] [PubMed] [Google Scholar]
- 10.Lipska KJ, Ross JS, Miao Y, Shah ND, Lee SJ, Steinman MA. Potential overtreatment of diabetes mellitus in older adults with tight glycemic control. JAMA internal medicine. 2015 Mar 1;175(3):356–362. doi: 10.1001/jamainternmed.2014.7345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Centers for Disease Control and Prevention (CDC). National Center for Health Statistics (NCHS) National Health and Nutrition Examination Survey, 1999–2012. Hyattsville, MD: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention; 1999–2012. [Google Scholar]
- 12.Centers for Disease Control and Prevention (CDC). National Center for Health Statistics (NCHS) National Health and Nutrition Examination Survey Examination Protocol, 1999–2012. Hyattsville, MD: Department of Health and Human Services, Centers for Disease Control and Prevention; 2008. [Google Scholar]
- 13.Centers for Disease Control and Prevention (CDC). National Center for Health Statistics (NCHS) National Health and Nutrition Examination Survey Laboratory Protocol: 1999–2012. Hyattsville, MD: U.S. Department of Health and Human Services, Centers for Disease Control and Prevention; 2008. [Google Scholar]
- 14.Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009 May 5;150(9):604–612. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. Journal of general internal medicine. 2001 Sep;16(9):606–613. doi: 10.1046/j.1525-1497.2001.016009606.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Centers for Disease Control and Prevention (CDC). National Center for Health Statistics (NCHS) National Health and Nutrition Examination Survey Examination Protocol. Hyattsville, MD: Department of Health and Human Services, Centers for Disease Control and Prevention; 2008. [Google Scholar]
- 17.Standards of medical care in diabetes--2015. Diabetes Care. 2014 Jan;37(Suppl 1):S14–80. doi: 10.2337/dc14-S014. [DOI] [PubMed] [Google Scholar]
- 18.Moreno G, Mangione CM, Kimbro L, Vaisberg E. Guidelines abstracted from the American Geriatrics Society Guidelines for Improving the Care of Older Adults with Diabetes Mellitus: 2013 update. Journal of the American Geriatrics Society. 2013 Nov;61(11):2020–2026. doi: 10.1111/jgs.12514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lipska KJ, Ross JS, Wang Y, et al. National trends in US hospital admissions for hyperglycemia and hypoglycemia among Medicare beneficiaries, 1999 to 2011. JAMA internal medicine. 2014 Jul;174(7):1116–1124. doi: 10.1001/jamainternmed.2014.1824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Budnitz DS, Lovegrove MC, Shehab N, Richards CL. Emergency hospitalizations for adverse drug events in older Americans. N Engl J Med. 2011 Nov 24;365(21):2002–2012. doi: 10.1056/NEJMsa1103053. [DOI] [PubMed] [Google Scholar]
- 21.Office of Disease Prevention and Health Promotion, editor. United States Department of Health and Human Services. Washington, D.C.: 2014. National Action Plan for Adverse Drug Event Prevention. [Google Scholar]
- 22.Department of Veterans Affairs, The Department of Defense. VA/DoD Clinical Practice Guideline For The Management Of DIiabetes Mellitus. 2010 [Google Scholar]
- 23.Seaquist ER, Anderson J, Childs B, et al. Hypoglycemia and diabetes: a report of a workgroup of the American Diabetes Association and the Endocrine Society. Diabetes Care. 2013 May;36(5):1384–1395. doi: 10.2337/dc12-2480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Huang ES, Laiteerapong N, Liu JY, John PM, Moffet HH, Karter AJ. Rates of complications and mortality in older patients with diabetes mellitus: the diabetes and aging study. JAMA internal medicine. 2014 Feb 1;174(2):251–258. doi: 10.1001/jamainternmed.2013.12956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zeitler P, Hirst K, Pyle L, et al. A clinical trial to maintain glycemic control in youth with type 2 diabetes. N Engl J Med. 2012 Jun 14;366(24):2247–2256. doi: 10.1056/NEJMoa1109333. [DOI] [PMC free article] [PubMed] [Google Scholar]