Fig. 6.
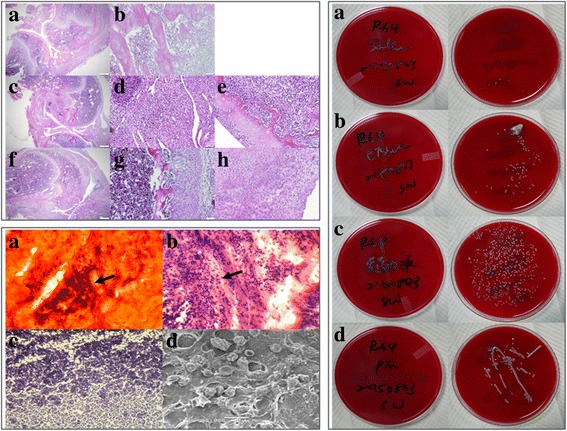
Histological, histopathological staining and microbiology assessment from osteomyelitis rats after 40 days infection. Left upper: Histological sections with H&E staining. Upper: Uninfected group, magnification a 40 × and b 1000 ×; Middle: Osteomyelitis 10 days after induction of infection, magnification c 40 ×, d and e 1000 ×; Lower: Osteomyelitis 40 days after induction of infection, magnification f 40 ×, g and h 1000 ×. In this figure, uninfected group possesses normal trabecular patterns of cancellous bone (upper: a and b); while in the acute suppurative changes of osteomyelitis, there is devitalized lamellar bone with scalloped edges and absence of stainable osteocytes and osteoblasts, edema and granulocytic infiltration of surrounding tissues are obvious (middle: c-e). In the chronic osteomyelitis, there is the irregular fragment of devitalized bone surrounded by dense fibrous tissue heavily infiltrated by plasma cells, lymphocytes, and only a few granulocytes (lower: f-h). Left lower: Histopathological staining. a Cango red staining, b H&E staining, c Gram staining and d SEM morphology, respectively. As shown here, GRAM-staining revealed the presence of bacteria within the defect site as Gram (+) Staphylococcus aureus bacteria could be detected (b and c). Cango red staining (a) and SEM image (d) showed the biofilm forming completed after 40 days induction of osteomyelitis. Magnification of pictures are 1000 ×. Right: Microbiology assessment from osteomyelitis rats after 40 days infection. Specimens streaked onto agar plate for: a Sterilized PBS as control group; b Tissue from the affected area; c Intramedullary liquid from the affected femur; d Implants from the affected animals. All smears taken from the experimental sites showed characteristic accretion of S. aureus infection. In the control group, there is few characteristic CFUs on the agar plate (a). On the other hand, tissue from the affected area, intramedullary liquid from the infected femur and the implants from the infected animals all showed characteristic growth of CFUs on agar plates (b-d)
