FIGURE 2.
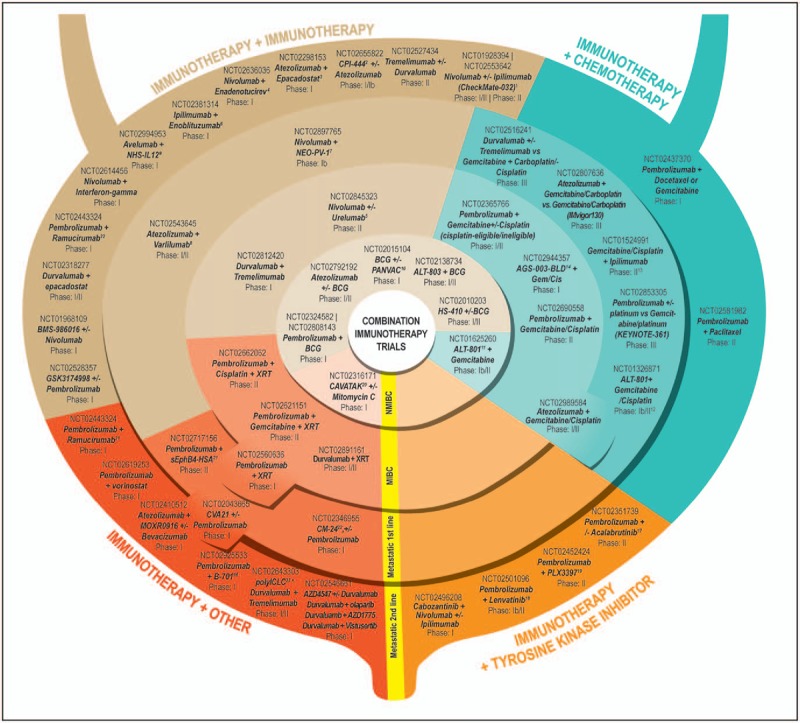
Immunotherapy Combination Clinical Trials in various stages of development utilizing immune therapies in combination with other immune therapies, chemotherapy, tyrosine kinase inhibitors, and other agents. Trials are further subdivided by disease state, into nonmuscle-invasive bladder cancer, muscle-invasive bladder cancer, metastatic first line, and metastatic second line. 1Objective response rate 38% for Nivolumab 1 mg/kg + Ipilimumab 3 mg/kg (n = 26; complete response 3.8%). Objective response rate 26% for Nivolumab 3 mg/kg + Ipilimumab 1 mg/kg (n = 104; complete response 2.9%). SITC 2016. 2Immune checkpoint inhibitor of the adenosine A2A receptor. 3Oral inhibitor of indoleamine 2,3-dioxygenase 1. 4Oncolytic group B adenovirus vaccine. 5mAb against CD137 receptor. 6mAb against B7-H3. 7Personalized cancer vaccine. 8mAb against CD27. 9Antibody-cytokine conjugate consisting of two heterodimers of IL-12, fused to a human mAb that has affinity for both single-stranded and double-stranded DNA. 10Poxvirus-based vector vaccine that induces tumor-specific immune response in T cells. 11IL-2 genetically fused to a humanized soluble T-cell receptor directed against the p53-derived peptides expressed on tumor cells. 12Objective response rate in five chemo-naïve patients 100% (two complete responses and three partial responses) and in five previously treated patients 60% (one complete response and two partial responses), for an overall objective response rate of 80% (three complete responses, five partial responses, 1 stable disease, and 1 progressive disease). Fishman et al.[22]. 13Objective response rate 64% (partial response 50%; complete response 14%); Galsky et al.[23]. 14Autologous dendritic cell-based immunotherapy. 15mAb against FGFR3. 16Second-generation Bruton's tyrosine kinase inhibitor. 17Tyrosine kinase inhibitor of VEGFR2. 18Tyrosine kinase inhibitor of KIT, CSF1R, and FLT3. 19Formulation of coxsackievirus type A21. 20Recombinant protein inhibitor of tumor growth and angiogenesis, complete response in one of three patients in higher dose cohort. https://www.viralytics.com/wp-content/uploads/2014/05/160315-EAU-CANON.pdf. 21mAb against VEGFR2. 22Humanized IgG4 (kappa) isotype mAb against CEACAM1. 23TLR3 agonist. BCG, Bacillus Calmette–Guérin; MIBC, muscle-invasive bladder cancer; NMIBC, nonmuscle-invasive bladder cancer; UC, urothelial carcinoma.
