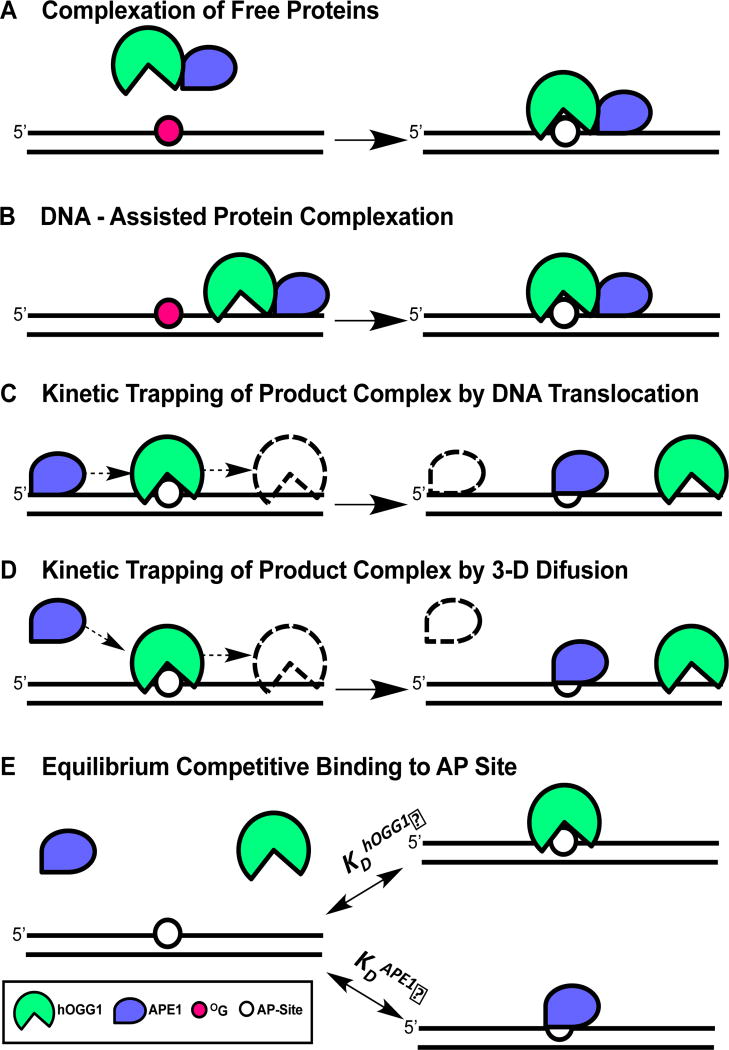
Figure 1. Possible mechanisms for displacement of hOGG1 from an AP site by APE1.
(A) Complexation of free proteins. In this mechanism, APE1 induces a conformational change in hOGG1 that facilitates turnover from the AP site. (B) DNA-facilitated protein complexation. In this mechanism, non-specific interactions of each protein with DNA are used to facilitate weak protein-protein interactions that facilitate release from the AP-site. (C) Kinetic trapping of the AP site by pre-association of APE1 with the DNA. (D) Kinetic trapping of the AP site by diffusional encounter with the hOGG1-AP site. (E) Equilibrium competitive binding of APE1 and hOGG1 to the AP product site. The experiments in this paper support the stimulation mechanisms shown in panels 1C and 1D (see Discussion).