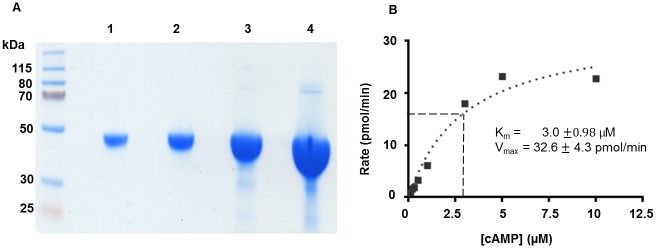
Fig 3. Purification and activity analysis of the recombinant catalytic domain of SmPDE4A.
(A) The three-step purification scheme described in the text resulted in purified His6-tagged SmPDE4A with the expected molecular mass of 44.2 kDa. Each lane (1–4) contains an increasing amount of protein (3, 6, 21 and 59 μg) demonstrating the absence of major contaminants. Molecular mass markers (in kDa) are indicated on the left. (B) Determination of Km and Vmax. Enzyme reaction rates were measured over increasing concentrations of the cAMP substrate up to 10 μM. All reactions ran for six minutes and contained 23.5 units/ml SmPDE4A. Km and Vmax values were determined by nonlinear regression analysis of the data (Prism GraphPad version. 6.03) using a Michaelis-Menten enzyme kinetics model. All data points were determined in triplicate.