Abstract
Th17 cells are critical effectors mediating the ocular surface autoimmunity in dry eye disease (DED). Increased IFN-γ has also been implicated in DED; however, it remains unclear to what extent Th1 cells contribute to DED pathogenesis. In this study, we investigated the cellular source of IFN-γ and assessed its contribution to corneal epitheliopathy in DED mice. We discovered a significant FigA+IFN-γ+ Th17/1 population and determined that these cells are derived from Th17 precursors. Adoptive transfer of Th17/1, but not Th1, cells confers the disease to naïve recipients as effectively as Th17 cells alone. DED-induced IL-12 and IL-23 are required for in vivo transition of pathogenic Th17 cells to IFN-γ-producers. Furthermore, using IFN-γ-deficient Th17 cells, we demonstrate the disease amplifying role of Th17-derived IFN-γ in DED pathogenesis. These results clearly demonstrate that Th17 cells mediate ocular surface autoimmunity through both IL-17A and IFN-γ.
INTRODUCTION
Dry eye disease (DED)3 is one of the most common ocular disorders for which patients seek care. Recent studies have demonstrated that ocular surface autoimmunity is the major underlying mechanism for DED. Increased levels of IL-17A and IFN-γ have been consistently observed in both clinical (1, 2, 3) and experimental DED (4, 5), suggesting that possibly both Th1 and Th17 cell responses are involved in DED pathogenesis. Furthermore, our group and others have demonstrated that Th17 cells are the principal effectors actively mediating DED, evidenced by (i) the presence of a prominent Th17 response in DED ocular surface and draining lymphoid tissues (4, 5, 6), (ii) specific resistance of Th17 cells, but not Th1 cells, to regulatory T cell (Treg) suppression (7), (iii) maintenance of disease chronicity by memory Th17 cells (8), and (iv) significant disease amelioration after neutralization of IL-17A (5, 7, 9).
Nevertheless, in spite of the dominant role of Th17 cells and their signature cytokine IL-17A in DED immunoinflammation, it has been shown that subconjunctival injection of exogenous IFN-γ to DED mice increases corneal epithelial apoptosis, while the corneal epithelium in IFN-γ KO mice is resistant to apoptosis (10), indicating a strong association of corneal epitheliopathy and increased IFN-γ in DED. Recently, we have shown that NK cells are the major source of IFN-γ during the innate immunity-dominant early acute DED stage, and depletion of NK cells or neutralization of IFN-γ in the early stage of DED reduces disease severity (11). However, these IFN-γ-producing NK cells gradually diminish after the initial stress (11), and hence cannot be the major producers for the abundant IFN-γ in late acute DED. The exact cellular source of the persisting IFN-γ and their role in DED pathogenesis thus remains to be determined.
In this study, for the first time, we examined the pathogenicity of multiple in vivo spontaneously developed T helper cell populations which secret IL-17A (Th17), IFN-γ (Th1), or both (Th17/1) in DED, and demonstrated that similar to Th17, Th17/1, but not Th1, cells are potently DED pathogenic. Furthermore, Th17 can convert to Th17/1 in vivo facilitated through IL-12 and IL-23 signaling, and such Th17-derived IFN-γ enhances ocular surface autoimmune response and thus amplifies DED severity.
MATERIALS AND METHODS
Animals
Female 6- to 8-week old wild-type (WT) C57BL/6 mice (Charles River Laboratories), B6.Rag1 knock out (KO) mice, and B6.IFN-γ KO mice (The Jackson Laboratory) were used for this study. All animal experiments were approved by the Schepens Eye Research Institute Animal Care and Use Committee, and adhered to the Association for Research in Vision and Ophthalmology Statement for the Use of Animals in Ophthalmic and Vision Research.
DED induction
DED was induced in mice as previously described (9). In brief, mice were placed in a controlled-environment chamber with a relative humidity below 20%, airflow of 15 L/min and a constant temperature of 21 to 23°C for 14 consecutive days. Thereafter, mice were transferred to the standard non-desiccated vivarium, where mice were maintained for an additional 4 months. Corneal epithelial disease was evaluated using fluorescein (Sigma-Aldrich) staining and scored using the National Eye Institute grading system (NEI, Bethesda, MD).
Histology
The whole eye ball was excised and fixed in 10% formalin for fixation. After dehydration, the specimens were embedded in methacrylate, cross-sectioned, and stained with hematoxylin and eosin. The morphology of the cornea and the conjunctiva was observed under a microscope (Nikon Eclipse E800) with a 40× objective.
Flow cytometry analysis
Conjunctivae tissues were first digested in RPMI (Invitrogen) with 2mg/ml DNase and 2mg/ml Collagenase (Roche) at 37°C. The following antibodies (Abs) were used for flow cytometry analysis: FITC-conjugated anti-CD3, FITC-conjugated anti-CD4, PerCP-Cy5.5- or APC-conjugated anti-IFN-γ (BioLegend), and PE-Cy7- or PE-conjugated anti- IL-17A (eBioscience). For intracellular IL-17A staining, cells were stimulated with 50 ng/mL phorbol 12-myristate 13-acetate and 500 ng/mL ionomycin (Sigma-Aldrich) for 6 hours at 37°C and 5% CO2 in the presence of GolgiStop™ (4 μl per 6 mL cell culture, BD Biosciences) to inhibit cytokine secretion. Stained cells were examined with an LSR II flow cytometer (BD Biosciences), and the results were analyzed using FlowJo software (Tree Star).
T cell adoptive transfer
Draining lymph node cells from DED mice were harvested and their CD4+ T cells were enriched via negative selection with CD4+ T cell isolation kit (Miltenyi Biotec Inc.). Thereafter, Th17 (IL-17A+IFN-γ−), Th17/1 (IL-17A+IFN-γ+), and Th1 (IL-17A−IFN-γ+) subsets were further sorted using IL-17A and IFN-γ cytokine secretion assay kits (Miltenyi Biotec Inc.) and a BD FACSAria™ sorter (BD Biosciences). Each of these subsets (2 × 104 cells) was subsequently injected intravenously into naïve B6.Rag1 KO mice, which were then placed in the CEC for 5 days. In the Ab treatment studies, sorted Th17 cells were injected intravenously into naïve B6.Rag1 KO mice, which were then placed in the CEC for 5 days. These recipient mice were injected intraperitoneally with 200 μg of anti-IL-12 (R & D Systems), 100 μg of anti-IL-23R (R & D Systems), 100 μg of anti-IL-12p40 Abs (R & D Systems), or 100 μg of isotype IgG (R & D Systems) one day before and one day after the cell transfer. Disease severity was evaluated using corneal fluorescein staining (CFS) described above.
Real-time PCR
Conjunctivae from mice were harvested, frozen in TRIzol® Reagent (Invitrogen Corp) and stored at −80°C until use. Total RNA was isolated with an RNeasy® Micro kit (Qiagen) according to the manufacturer’s recommendations and reverse transcribed using a SuperScript™ III kit (Invitrogen Corp). Real-time PCR was performed using TaqMan® Universal PCR Master Mix and predesigned primers for IFN-γ (Mm01168134_m1) and GAPDH (Mm99999915_g1) (Applied Biosystems) in a LightCycler® 480 II System (Roche Applied Science). The GAPDH gene was used as an endogenous control for each reaction. The results of quantitative PCR were analyzed by the comparative CT method in which the target change = 2−ΔΔCT. The results were normalized by the CT value of GAPDH, and the mean CT of relative mRNA level in the control IgG group was used as the calibrator.
ELISA
For protein extraction, draining lymph nodes were harvested and stored in cold sterile PBS containing protease inhibitors (Sigma-Aldrich) at −80°C until used. The samples were homogenized on ice and centrifuged. The supernatant was assayed using commercial ELISA kits for levels of the total protein (Thermo Scientific), IL-12, and IL-23 (eBioscience).
Statistical analyses
Mann-Whitney Test was used with the software Prism 5, and differences were considered significant at p < 0.05.
RESULTS
Presence of IL-17A+ IFN-γ+ T helper cells (Th17/1) in severe DED
Acute and chronic DED was induced using our well-established murine models, and disease severity peaked at acute Day 14 (Fig. 1A). The ocular surface showed characteristic change in corneal epithelial thickness and decreased numbers and atrophy of conjunctival goblet cells at Day 14 (Fig. 1B). We further investigated IFN-γ single producer (Th1), IL-17A single producer (Th17), and IL-17A and IFN-γ producer (Th17/1) at multiple time points by flow cytometry (Fig. 1C). In the eye-draining lymph nodes (DLN), Th17 cells occurred during the initial disease induction phase and continuously increased as the disease exacerbated. Following the emergence of Th17 cells, we observed double-positive Th17/1 cells as the disease severity exacerbated. When the disease entered a chronic stage (~4 months) the Th17 frequency decreased, but still persisted at high levels. These Th17 cells have been previously identified as memory Th17 cells which principally mediate the chronicity of DED (9). During this stage when DED is characterized as persisting low-grade inflammation on the ocular surface (9), the Th17/1 frequency diminished (Fig. 1D). When these chronic DED mice were re-challenged with desiccating stress, Th17/1 cells increased again, along with exacerbated disease (Supplemental Fig. 1). The same kinetic patterns of Th17 and Th17/1 cells were observed at the inflamed ocular surface (conjunctiva) (Fig. 1E). In contrast, Th1 cell frequencies remained unchanged throughout the course of DED (Fig. 1C).
Figure 1. Increased IFN-γ+Th17 cells (Th17/1) in severe dry eye disease (DED).
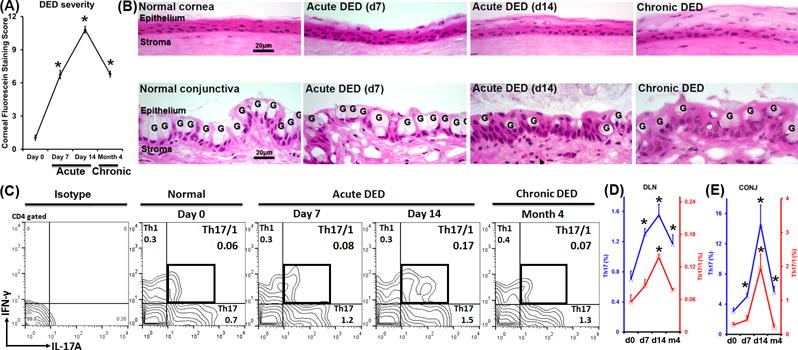
Frequencies of Th1, Th17, and Th17/1 cells were examined by flow cytometry throughout the course of DED (normal, acute, and chronic stages). (A) DED severity was evaluated by corneal fluorescein staining scores at different stages (n = 20 eyes per time point). *, p < 0.05 as compared to Day 0. (B) Pathological changes of corneal (upper panel) and conjunctival epithelium (lower panel) in different stages of DED. Ocular surface in DED is characterized by change in epithelial thickness in the cornea, and loss of goblet cells (marked as G) in the conjunctiva. (C) Representative flow cytometry plots show cell frequencies gated on CD4+ cells. (D) Kinetic changes of Th17 (blue line) and Th17/1 (red line) cells in eye-draining lymph nodes (DLN) during the course of disease are summarized as mean±SEM from one representative experiment out of three performed (n = 4 mice per time point per group). (E) Kinetic changes of the Th17 (blue line) and Th17/1 (red line) cells in conjunctivae (CONJ) are summarized as mean±SEM from all three experiments with 4–6 eye tissues pooled together as one flow cytometry sample (n = 4 samples per time point per group). *, p < 0.05 as compared to Day 0.
Th17/1 cells isolated from DED are capable of inducing ocular surface inflammation
The pathogenicity of Th17 cells in DED has been demonstrated previously by blocking IL-17A in vivo (5, 7, 9). In the present study, we adoptively transferred Th subsets from DED mice into T cell-deficient naïve Rag1 KO mice to determine their function. Th1, Th17, and Th17/1 cells were isolated (Supplemental Fig. 2) at day 14 after the development of severe DED, and were then intravenously transferred to Rag1 KO recipients, which were subsequently challenged with desiccating environmental stress for 5 days. Rag1 KO mice without adoptive transfer served as controls and showed no disease after 5 days of desiccating stress (mean disease score = 1.38 out of 15 points), confirming the central role of T cells in inducing corneal epitheliopathy in DED (Fig. 2A, 2B). Similarly, mice adoptively transferred with single-positive Th1 cells developed no disease as well. In contrast, both Th17 and Th17/1 recipients exhibited significantly higher disease scores than control or Th1 recipients. There were no significant differences in the disease severity between Th17 and Th17/1 recipients (Fig. 2A, 2B). We also found increased T cell infiltration in the conjunctivae of Th17 and Th17/1 recipients, but not in the control or Th1 recipients (Fig. 2C). No T cells were detected in the DLN as well in Th1 recipients (Supplemental Fig. 3), suggesting that Th1 cells isolated from DED were not disease-specific effectors and unable to migrate to target tissues.
Figure 2. Both Th17 and Th17/1, but not Th1 cells isolated from DED are pathogenic.
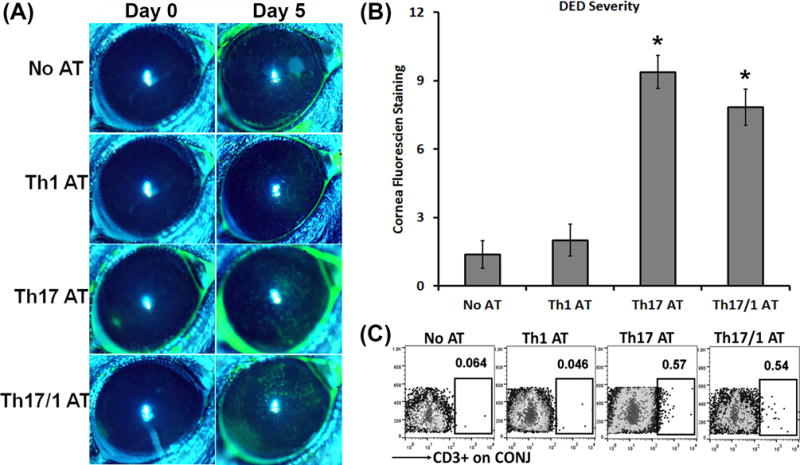
Severe DED was induced for 14 days in wild-type mice, and then Th1, Th17, and Th17/1 cells were isolated from draining lymph nodes. 1 × 104 cells from each subset were intravenously injected into Rag1 KO mice. Immediately after the adoptive transfer, these Rag1 KO mice were subject to desiccating stress for 5 days. AT, adoptive transfer. (A) Clinical disease severity was evaluated by corneal fluorescein staining and representative images show baseline (Day 0) and 5 days post-AT. (B) DED scores in each group at Day 5 are summarized as mean±SEM in bar graphs (n = 6–8 eyes per group). *, p < 0.05 as compared to No AT or Th1 AT group. (C) Representative flow cytometry dot plots from three separate experiments (4–6 eye tissues pooled together for each group) show increased infiltration of transferred T cells in the conjunctivae (CONJ) in mice with AT of Th17 and Th17/1 cells.
Pathogenic Th17 cells are plastic and convert into IFN-γ producers via support of IL-12 and IL-23
In vitro polarized Th17 cells have been shown to give rise to IFN-γ-producing cells after adoptive transfer in a murine model of colitis (12, 13). To investigate whether in vivo spontaneously generated pathogenic Th17 cells from DED have the similar plastic capacity developing into IL-17A+IFN-γ+ double-positive Th17/1 cells, we adoptively transferred IL-17A+ single-positive Th17 cells to Rag1 KO mice and induced DED. After 5 days we analyzed the draining lymph nodes for IFN-γ-producing T cells by flow cytometry. We found that ~50% of Th17 cells converted into IL-17A/IFN-γ double producers (Th17/1) after being adoptively transferred to the Rag1 KO mice (Fig. 3A, upper panel, and Fig. 3B). Meanwhile, the other group of naïve Rag1 KO mice receiving Th17 cells was housed in the normal environment without DED induction. After 5 days, a milder ocular surface epitheliopathy was developed (Fig. 3C) along with only ~20% Th17 cells becoming IFN-γ-producing cells (Fig. 3A, lower panel, and Fig. 3B). In addition, a substantial part of transferred Th17 cells lost the expression of IL-17A in both with DS (~30%) and without DS (~65%) groups, suggesting that subpopulations of purified DED-Th17 cells might have different capacity for stable IL-17A expression and additional IFN-γ acquisition. We further examined IL-12 and IL-23 cytokine levels in the draining lymph nodes. DED-induced recipients exhibited a significant 2-fold up-regulation of both IL-12 and IL-23 mRNA than those without subject to desiccating stress (Fig. 3D). Protein levels of these two cytokines in DED-induced recipients were also consistently increased (Fig. 3E).
Figure 3. Th17 cells isolated from DED convert into IFN-γ-secreting cells associated with DED-induced IL-12 and IL-23.
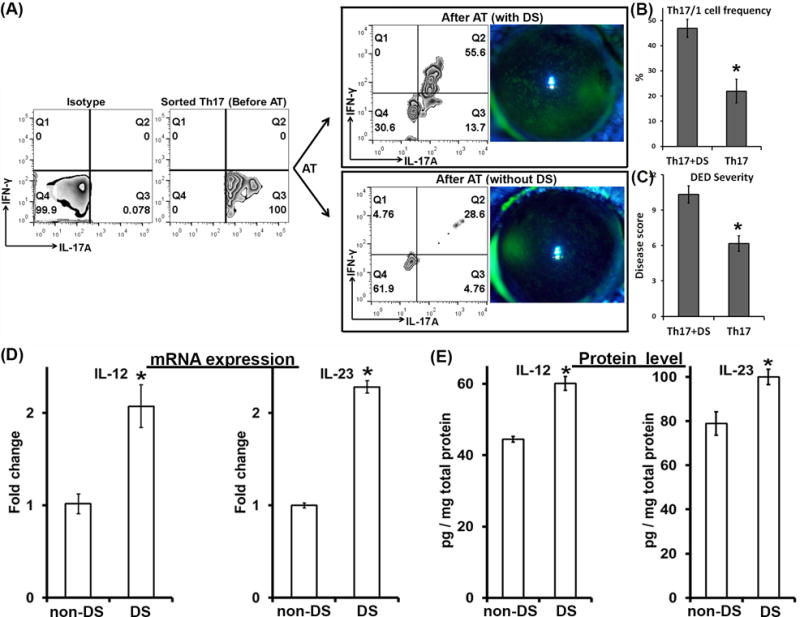
DED-specific Th17 cells were isolated from severe DED (Before AT) and then adoptively transferred into Rag1 KO mice which were then subject to desiccating stress (DS) or not (non-DS) for 5 days. AT, adoptive transfer. (A) Clinical disease severity was evaluated by corneal fluorescein staining and representative images were shown 5 days post-AT. In addition, eye-draining lymph nodes of the recipient mice were collected and analyzed for IFN-γ and IL-17A expressions by flow cytometry (After AT). The data were summarized as mean±SEM for Th17/1 frequency (n = 3 mice) (B) and disease score (n = 6 eyes) (C) from one experiment out of two performed. *, p < 0.05. (D) mRNA and (E) protein expressions of IL-12 and IL-23 in the draining lymph nodes were quantified by real-time RT-PCR and ELISA, respectively. *, p < 0.05. Six eyes from 3 mice in each group were analyzed.
IL-12 and IL-23 are required for Th17 conversion to IFN-γ-producers
We next delineated whether the DED-induced IL-12 and IL-23 are responsible for the acquisition of IFN-γ in DED-pathogenic Th17 cells. It has been reported that both IL-12 and IL-23 are essential for enhanced IFN-γ production by in vitro-polarized Th17 cells (12, 14, 15), and Th17 cells from IL-23p19-deficient IL-17A reporter mice completely lack IL-17A/IFN-γ double-positive or IFN-γ single-positive cells (16). To determine the roles of IL-12 and IL-23 in IFN-γ secretion in in vivo induced, disease-specific Th17 cells, we isolated Th17 cells from DED mice and adoptively transferred them into Rag1 KO mice. The recipient mice were challenged with desiccating environmental stress for 5 days and treated with anti-IL12, anti-IL23R, anti-IL12p40 Abs, or control IgG one day before and one day after the transfer of Th17 cells. All three Ab treatments led to a substantial reduction of IFN-γ+ T cells, especially IL-17A+IFN-γ+ T cells. Anti-IL23R and anti-IL12p40 treatments resulted in the most significant reduction of IL-17A+IFN-γ+ T cells (Fig. 4A). Correspondingly, the development of ocular surface inflammation was significantly delayed and disease severity was dramatically decreased in all treated groups (Fig. 4B). Furthermore, diminished disease correlated with significantly lower ocular surface IFN-γ levels in Ab-treated Th17 recipients (Fig. 4C).
Figure 4. IL-12 and IL-23 mediate the conversion of Th17 cells into IFN-γ-secreting cells.
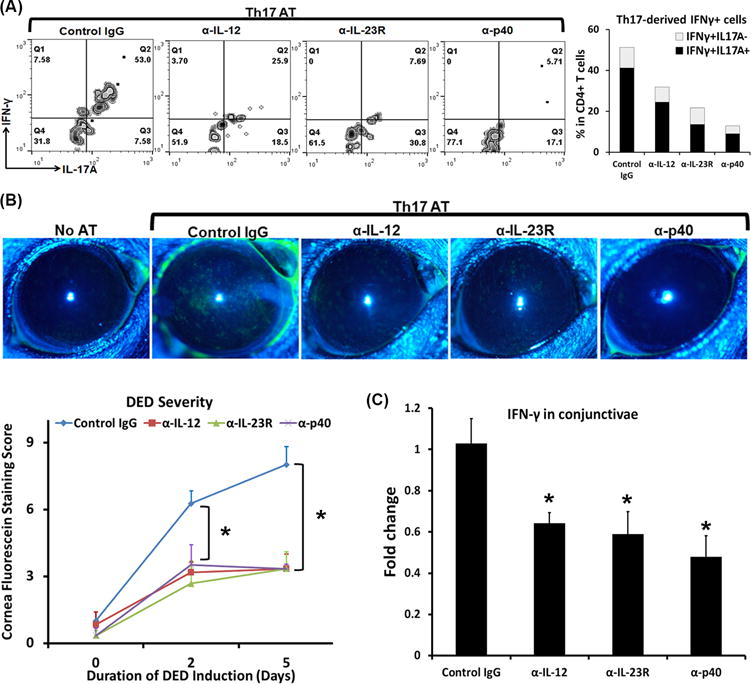
DED-specific Th17 cells were isolated and adoptively transferred to Rag1 KO mice, which were then subject to desiccating stress for 5 days. These mice were injected with anti-IL12, anti-IL23R, anti-IL12p40 Abs, or control IgG one day before and one day after the transfer of Th17 cells. AT, adoptive transfer. (A) Eye-draining lymph nodes were collected and analyzed for IFN-γ-expressing Th17 cells. Representative flow cytometry graphs gated on CD4+ are shown on the left, and the IFN-γ+IL-17A− and IFN-γ+IL-17A+ cell frequencies are summarized as mean on the right bar graphs. Anti-IL12, anti-IL23R, and anti-IL12p40 Ab-treated groups exhibited significantly decreased IFN-γ+IL-17A+ cells (24.5±0.8%, 13.6±6.0%, and 9.0±1.9%, respectively) as compared to control IgG group (41.2±5.9%, p < 0.05). Furthermore, both anti-IL23R and anti-IL12p40-treated groups showed even lower IFN-γ+IL-17A+ cell frequencies than anti-IL12-treated group (p < 0.05). No significant differences between anti-IL23R and anti-IL12p40-treated groups. (B) Clinical disease severity was evaluated by corneal fluorescein staining with the representative images exhibited. (C) IFN-γ mRNA expression in the conjunctivae was quantified by real-time RT-PCR, and data are shown as relative changes to control IgG-treated mice. *, p < 0.05 as compared to control IgG group. n = 6-8 eyes/group.
IFN-γ secreted by Th17 cells contributes to DED severity
Th17 plasticity plays important functions in autoimmune disease pathogenesis, and has even been considered indispensable for efficient disease induction in several forms of autoimmunity (13, 16). To examine whether Th17-derived IFN-γ is required for the development of DED, we induced DED in IFN-γ KO mice and then isolated their Th17 cells. Th17 cells from IFN-γ KO DED showed comparable expression levels of IL-17A as Th17 cells from WT DED (Fig. 5A). Subsequently, these cells were adoptively transferred to Rag1 KO mice. Compared to recipients of WT DED Th17 cells, those receiving IFN-γ KO DED Th17 cells developed significantly milder disease (Fig. 5B, 5C). Correspondingly, T cells recovered from draining lymph nodes of IFN-γ KO Th17 recipients showed no IFN-γ expression (Fig. 5D). These data demonstrated that Th17 plasticity with the capability of producing IFN-γ is required for their enhanced pathogenicity in DED.
Figure 5. IFN-γ KO Th17 cells leads to less severe disease.
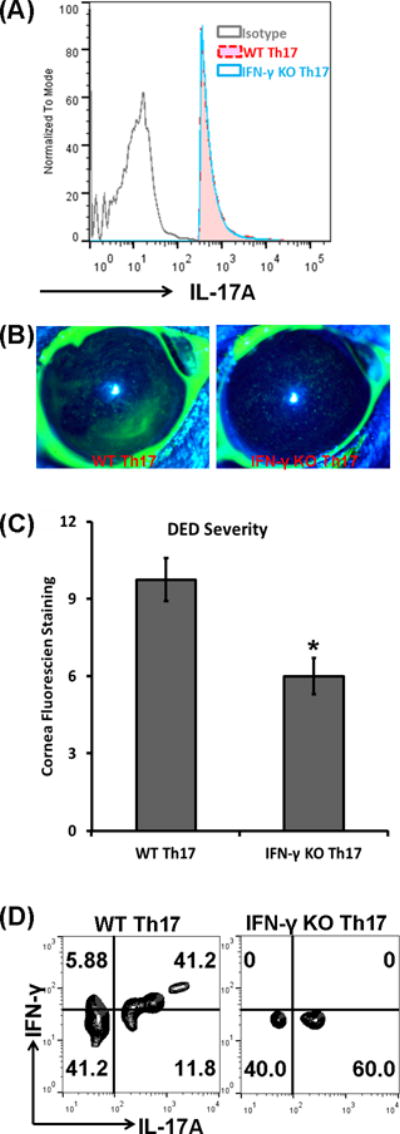
DED was induced in wild-type (WT) and IFN-γ KO mice, and then Th17 cells were isolated and adoptively transferred into Rag1 KO mice, which were then subject to the desiccating stress for 5 days. (A) IL-17A expression levels were assessed in Th17 cells from draining lymph nodes of WT and IFN-γ KO DED mice using flow cytometry. Sorted WT Th17 and IFN-γ KO Th17 cells from DED expressed comparable levels of IL-17A. (B) Representative corneal fluorescein staining images of mice receiving WT Th17 cells or IFN-γ KO Th17. (C) Mice receiving IFN-γ KO Th17 cells showed significantly reduced disease severity than those receiving WT Th17 cells. *, p < 0.05. (D) The expressions of IFN-γ and IL-17A by the transferred Th17 cells were analyzed by flow cytometry and representative plots are shown. n = 6–8 eyes in each group.
DISCUSSION
The current study shows that in addition to the well-known pathogenic cytokine IL-17A, IFN-γ derived from Th17 cells also amply contributes to ocular surface epitheliopathy and autoimmune inflammation in DED. Previously, we have reported that activated NK cells are the major source of IFN-γ during the early acute phase of DED; however, it has remained unclear whether a Th1 response contributes to DED progression via its secreted IFN-γ as well. Here, we investigated the pathogenicity of different T helper subsets in DED and found that Th1 cells isolated from DED cannot induce disease due to their inability to migrate to the ocular surface. In contrast, both IL-17A+ single-positive Th17 cells and IL-17A+IFN-γ+ double-positive Th17/1 cells migrate to the ocular surface where they contribute to DED pathology. Further, the conversion of Th17 cells to Th17/1 cells requires microenvironmental IL-12 and IL-23, and the IFN-γ produced by Th17 cells is essential and indispensable for the severe ocular surface immune response, suggesting that Th17 cells drive DED via both IL-17A and IFN-γ.
In the past several years, the critical role of IL-17A+ T helper cells in ocular surface autoimmunity in DED has been reported (5, 7, 8). On the other hand, although increased IFN-γ has been consistently reported in DED (1, 3, 4, 5), its precise role in DED immunopathogenesis has remained incompletely understood. The major hurdle lay in the cellular source of IFN-γ during disease progression since NK and Th1 cell numbers are not significantly increased in DED (4, 6, 11). Recently, the classic notion that diverse T helper cell subsets are terminally differentiated has been revised; due to their plasticity Th17 cells can acquire IFN-γ expression in vitro (12). In agreement with previous reports (4, 5, 8), our current study found persistently increased Th17 but not Th1 cells in both DED ocular surface and draining lymph nodes. For the first time, we discovered IL-17A+IFN-γ+ double-positive Th17/1 cells emerging from Th17 cells upon disease induction. Our data suggest that plastic Th17 cells serve as a major source of IFN-γ during DED progression and demonstrate that increased IFN-γ expression is part of the pathogenic Th17 response in severe DED, and thus reconcile existing data on the role of IFN-γ in DED (1, 3, 4, 5). When the disease enters its chronic stage characterized by persistent, low-grade inflammation, double-positive Th17/1 cells diminish and memory Th17 cells represent the predominant pathogenic effectors (8). However, upon re-challenge with desiccating environmental stress, Th17/1 cells increase again in these chronic DED and correlate with exacerbated disease (Supplemental Fig. 1). Similarly, in a bacterial infection model, vaccination-induced memory Th17 cells became Th1-like cells after infection with the bacteria (17). In vivo transfer of pathogenic Th17 cells proves their ability to secrete IFN-γ and induce immune damages in the target tissues, suggesting that the critical pathogenic role of Th17 in ocular surface autoimmunity correlates with both IL-17A and IFN-γ. Th17/1 cells, upon adoptive transfer, are able to infiltrate the ocular surface and induce DED comparable to Th17 cells. In contrast, adoptive transfer of Th1 cells into immunodeficient mice showed no ocular infiltration of T cells nor induced DED, demonstrating that these single-positive Th1 cells cannot specifically migrate to the ocular surface to induce DED. Migration of Th17, and most likely also Th17/1cells to the ocular surface is mediated through their expression of CCR6 (6, 18) and the enriched CCL20 environment at the ocular surface in DED (6).
We also found that the in vivo conversion of DED-Th17 cells to IFN-γ-producing cells was associated with the increased microenvironmental IL-12 and IL-23 levels. It has been reported that in vitro treatment of polarized Th17 cells with IL-12 or IL-23 promotes their IFN-γ secretion (12, 19), which is dependent on the transcription factors STAT4 and T-bet (12). In contrast to existing studies on in vitro-generated non-specific Th17 cells (12, 13, 14, 15), we adoptively transferred disease-specific Th17 cells into naïve recipients, and then evaluated the effect of IL-12 and IL-23 blockade on their acquisition of IFN-γ. DED-Th17 recipients treated with anti-IL-12, anti-IL-23R, or anti-IL12p40 exhibited a significantly less disease along with decreased frequencies of either IL-17A+IFN-γ+ or IL-17A−IFN-γ+ T cells, as well as decreased IFN-γ levels at the ocular surface. These findings demonstrate that both IL-12 and IL-23 are essential to Th17 plasticity and pathogenicity in vivo.
Furthermore, adoptive transfer of DED-Th17 cells from IFN-γ KO mice to T cell-deficient recipients causes DED corneal epitheliopathy. Although in the recipients, there are other IFN-γ-producing cells than T cells, such as NK cells, they do not develop DED without T cells transfer (Fig. 2), and thus the disease observed is due to the pathogenic roles of the transferred IFN-γ KO Th17 cells. However, the damage is less severe than that caused by WT DED-Th17 cells, demonstrating an important contribution of Th17-derived IFN-γ to DED pathogenesis. It has been reported that Th17 plasticity is indispensable for development of experimental autoimmune encephalomyelitis (EAE) (16), colitis (13), and diabetes (19). In contrast, one study has shown that stable Th17 cells, which are derived from IL-12 KO mice, are capable of inducing the same severe EAE as plastic Th17 cells (20). Our results demonstrate that Th17 plasticity enhances Th17 pathogenicity and is required for a severe ocular surface immunoinflammation. Therefore, the specific roles of Th17-derived IFN-γ and IL-17A in autoimmunity may be disease and context dependent.
In summary, our data demonstrate an important role of IFN-γ-secreting Th17 cells, but not conventional Th1 cells, in amplifying ocular surface autoimmunity. Thus, we reveal a previously undefined mechanism of Th17-derived IFN-γ in DED, highlighting the importance of blocking both Th17-dervied IL-17A and IFN-γ in designing clinical strategies that target DED. Because IFN-γ secretion by Th17 cells in DED is driven by IL-12 and IL-23, blocking IL-12p40 (shared subunit of IL-12 and IL-23) may prove to be an effective strategy to reduce Th17 cell pathogenicity in DED, the most common ocular pathology.
Supplementary Material
Acknowledgments
We thank Dr. Susanne Eiglmeier for critical reading and editing of this manuscript.
Footnotes
This work was supported by the National Institutes of Health (NIH) grant EY20889 (to RD).
Abbreviations used in this paper: Ab, antibody; CONJ, conjunctivae; DED, Dry eye disease; DLN, draining lymph node; KO, knockout; Th17/1, IL-17A+IFN-γ+CD4+ T cells; WT, wild-type.
References
- 1.Massingale ML, Li X, Vallabhajosyula M, Chen D, Wei Y, Asbell PA. Analysis of inflammatory cytokines in the tears of dry eye patients. Cornea. 2009;28:1023–1027. doi: 10.1097/ICO.0b013e3181a16578. [DOI] [PubMed] [Google Scholar]
- 2.Kang MH, Kim MK, Lee HJ, Lee HI, Wee WR, Lee JH. Interleukin-17 in various ocular surface inflammatory diseases. J Korean Med Sci. 2011;26:938–944. doi: 10.3346/jkms.2011.26.7.938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Meadows JF, Dionne K, Nichols KK. Differential profiling of T-cell cytokines as measured by protein microarray across dry eye subgroups. Cornea. 2016;35:329–335. doi: 10.1097/ICO.0000000000000721. [DOI] [PubMed] [Google Scholar]
- 4.Chen Y, Chauhan SK, Lee HS, Stevenson W, Schaumburg CS, Sadrai Z, Saban DR, Kodati S, Stern ME, Dana R. Effect of desiccating environmental stress versus systemic muscarinic AChR blockade on dry eye immunopathogenesis. Invest Ophthalmol Vis Sci. 2013;54:2457–2464. doi: 10.1167/iovs.12-11121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.De Paiva CS, Chotikavanich S, Pangelinan SB, Pitcher JD, 3rd, Fang B, Zheng X, Ma P, Farley WJ, Siemasko KF, Niederkorn JY, Stern ME, Li DQ, Pflugfelder SC. IL-17 disrupts corneal barrier following desiccating stress. Mucosal Immunol. 2009;2:243–253. doi: 10.1038/mi.2009.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dohlman TH, Chauhan SK, Kodati S, Hua J, Chen Y, Omoto M, Sadrai Z, Dana R. The CCR6/CCL20 axis mediates Th17 cell migration to the ocular surface in dry eye disease. Invest Ophthalmol Vis Sci. 2013;54:4081–4091. doi: 10.1167/iovs.12-11216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chauhan SK, El Annan J, Ecoiffier T, Goyal S, Zhang Q, Saban DR, Dana R. Autoimmunity in dry eye is due to resistance of Th17 to Treg suppression. J Immunol. 2009;182:1247–1252. doi: 10.4049/jimmunol.182.3.1247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen Y, Chauhan SK, Lee HS, Saban DR, Dana R. Chronic dry eye disease is principally mediated by effector memory Th17 cells. Mucosal Immunol. 2014;7:38–45. doi: 10.1038/mi.2013.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chauhan SK, Jin Y, Goyal S, Lee HS, Fuchsluger TA, Lee HK, Dana R. A novel pro-lymphangiogenic function for Th17/IL-17. Blood. 2011;118:4630–4634. doi: 10.1182/blood-2011-01-332049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang X, Chen W, De Paiva CS, Volpe EA, Gandhi NB, Farley WJ, Li DQ, Niederkorn JY, Stern ME, Pflugfelder SC. Desiccating stress induces CD4+ T-cell-mediated Sjögren’s syndrome-like corneal epithelial apoptosis via activation of the extrinsic apoptotic pathway by interferon-γ. Am J Pathol. 2011;179:1807–1814. doi: 10.1016/j.ajpath.2011.06.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen Y, Chauhan SK, Saban DR, Sadrai Z, Okanobo A, Dana R. Interferon-γ-secreting NK cells promote induction of dry eye disease. J Leukoc Biol. 2011;89:965–872. doi: 10.1189/jlb.1110611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lee YK, Turner H, Maynard CL, Oliver JR, Chen D, Elson CO, Weaver CT. Late developmental plasticity in the T helper 17 lineage. Immunity. 2009;30:92–107. doi: 10.1016/j.immuni.2008.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Harbour SN, Maynard CL, Zindl CL, Schoeb TR, Weaver CT. Th17 cells give rise to Th1 cells that are required for the pathogenesis of colitis. Proc Natl Acad Sci USA. 2015;112:7061–7066. doi: 10.1073/pnas.1415675112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mukasa R, Balasubramani A, Lee YK, Whitley SK, Weaver BT, Shibata Y, Crawford GE, Hatton RD, Weaver CT. Epigenetic instability of cytokine and transcription factor gene loci underlies plasticity of the T helper 17 cell lineage. Immunity. 2010;32:616–627. doi: 10.1016/j.immuni.2010.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang Y, Godec J, Ben-Aissa K, Cui K, Zhao K, Pucsek AB, Lee YK, Weaver CT, Yagi R, Lazarevic V. The transcription factors T-bet and Runx are required for the ontogeny of pathogenic interferon-γ-producing T helper 17 cells. Immunity. 2014;40:355–366. doi: 10.1016/j.immuni.2014.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hirota K, Duarte JH, Veldhoen M, Hornsby E, Li Y, Cua DJ, Ahlfors H, Wilhelm C, Tolaini M, Menzel U, Garefalaki A, Potocnik AJ, Stockinger B. Fate mapping of IL-17-producing T cells in inflammatory responses. Nat Immunol. 2011;12:255–263. doi: 10.1038/ni.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lindenstrøm T, Woodworth J, Dietrich J, Aagaard C, Andersen P, Agger EM. Vaccine-induced Th17 cells are maintained long-term postvaccination as a distinct and phenotypically stable memory subset. Infect Immun. 2012;80:3533–3544. doi: 10.1128/IAI.00550-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Coursey TG, Gandhi NB, Volpe EA, Pflugfelder SC, de Paiva CS. Chemokine receptors CCR6 and CXCR3 are necessary for CD4(+) T cell mediated ocular surface disease in experimental dry eye disease. PLoS One. 2013;8:e78508. doi: 10.1371/journal.pone.0078508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yoon KC, Park CS, You IC, Choi HJ, Lee KH, Im SK, Park HY, Pflugfelder SC. Expression of CXCL9, -10, -11, and CXCR3 in the tear film and ocular surface of patients with dry eye syndrome. Invest Ophthalmol Vis Sci. 2010;51:643–650. doi: 10.1167/iovs.09-3425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Carbajal KS, Mironova Y, Ulrich-Lewis JT, Kulkarni D, Grifka-Walk HM, Huber AK, Shrager P, Giger RJ, Segal BM. Th cell diversity in experimental autoimmune encephalomyelitis and multiple sclerosis. J Immunol. 2015;195:2552–2559. doi: 10.4049/jimmunol.1501097. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


