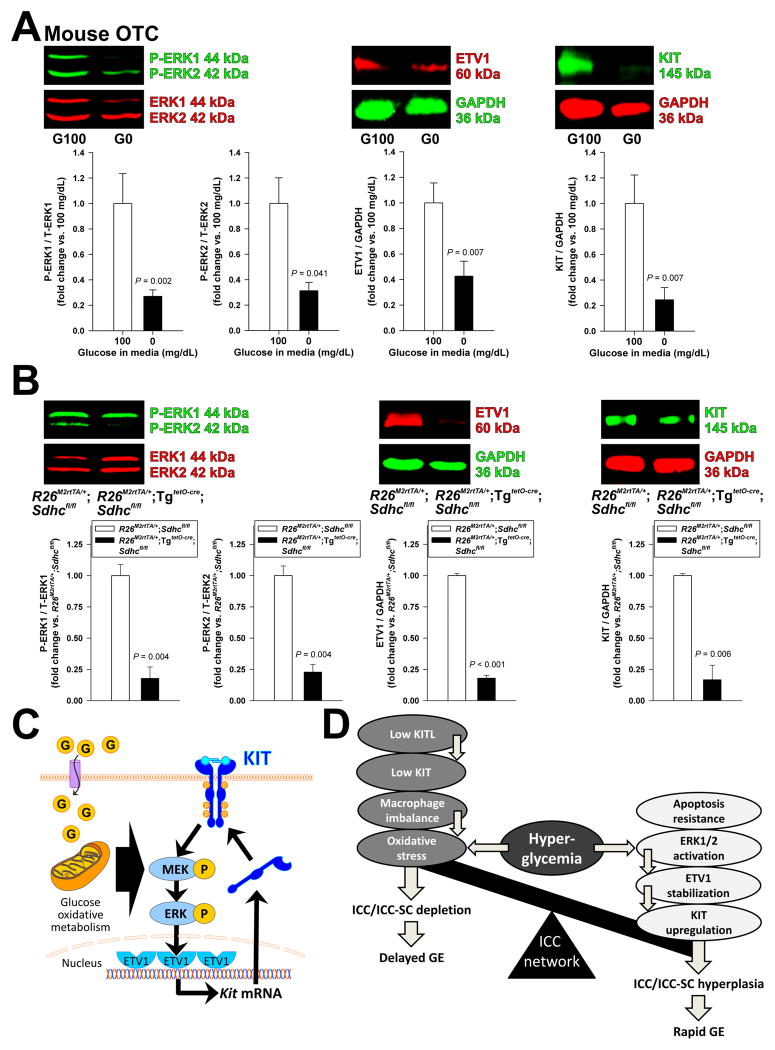
Figure 7.
Glucose oxidative metabolism regulates ICCs through the ERK-ETV1-KIT pathway. (A) Glucose deprivation (0 mg/dL; G0) for 48 h inhibited ERK1-ERK2 phosphorylation and reduced ETV1 and KIT protein expression in OTCs from gastric tunica muscularis of PND 14–16 C57BL/6J mice compared to 100 mg/dL glucose (G100) (n=8/group). (B) Effects of conditional genomic deletion of Sdhc on ERK1-ERK2 phosphorylation and ETV1 and KIT protein levels analyzed by WB in the gastric corpus+antrum tunica muscularis of doxycyclin-treated R26M2rtTA/+;TgtetO-cre;Sdhcfl/fl and R26M2rtTA/+;Sdhcfl/fl controls. Sdhc deletion reduced ERK1-ERK2 phosphorylation and ETV1 and KIT protein levels (n=5–6/group). (C) Proposed mechanism of glucose-induced KIT upregulation. Glucose (G) activates ERK1-ERK2 phosphorylation (P) in a manner dependent on oxidative metabolism. ERK1-ERK2 activation increases ETV1 protein by preventing its proteasomal degradation.6,8 ETV1 then activates KIT transcription.7,8 Increased KIT protein levels reinforce the signaling loop. (D) Proposed mechanisms regulating ICC numbers and gastric emptying. ICCs are reduced and gastric emptying is delayed by reduced KITL and KIT levels and macrophage imbalance facilitating oxidative stress from hyperglycemia. ICCs are increased and gastric emptying is accelerated by hyperglycemia-induced ERK1-ERK2 phosphorylation, ETV1 stabilization and KIT upregulation. These changes are facilitated by the ICCs’ resistance to hyperglycemia-induced apoptosis. The balance of the opposing outcomes of hyperglycemia determines ICC numbers and gastric emptying in diabetes.