Figure 6.
Opening of the Smc Rod Is a Prerequisite for Chromosomal Targeting
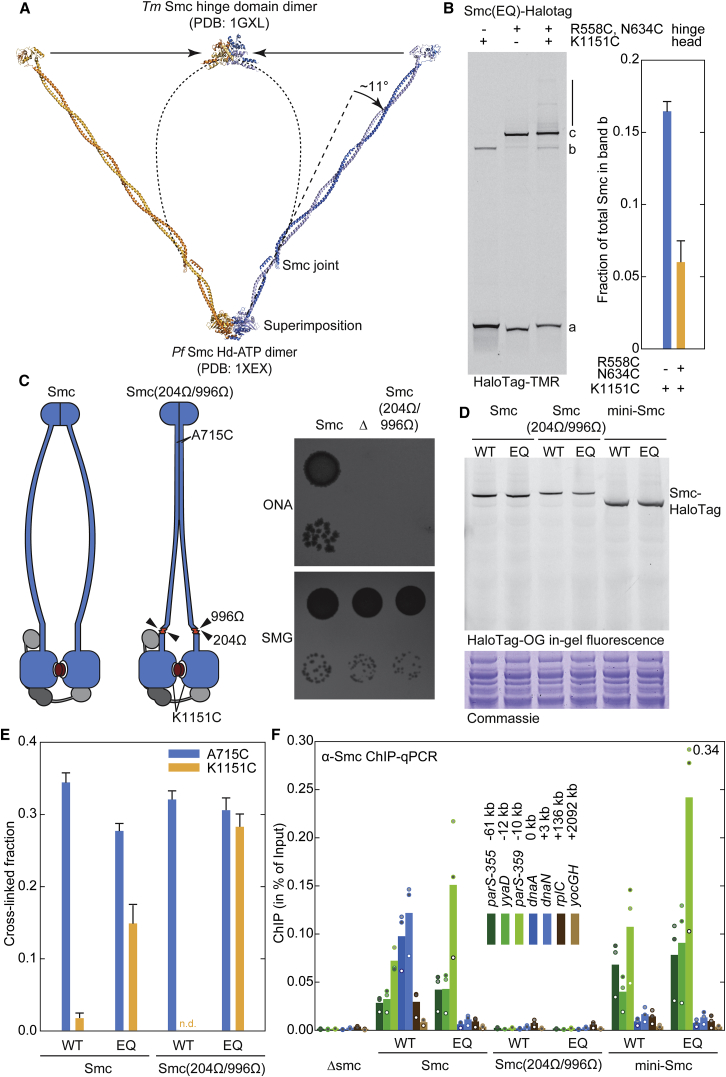
(A) Superimposition of two Smc monomers taken from the reconstructed Smc rod (Figure 4B) with an ATP dimer structure of the Pf Smc head (PDB: 1XEX). Significant bending of the coiled coils is required to generate a closed ring-shaped Smc dimer. The Smc joint bends the Smc coiled coil away from the central symmetry axis by about 11°.
(B) ATP engagement of Smc heads in Smc proteins with intact hinge dimers. Smc(EQ)-HaloTag cells with cysteines at the hinge and/or head were cross-linked by BMOE. HaloTag protein was labeled by Halo-TMR and analyzed by SDS-PAGE (left). Smc monomer (a), head-head dimer (b), and hinge-hinge dimer (c) bands are marked. The vertical line denotes additional species appearing in the presence of hinge and head cysteines. These likely represent circular species as well as larger oligomers. Please note that the contrast is enhanced to display low-abundance species in the figure. Quantification of the fraction of Smc protein in the head-head band (b) is shown (right). Mean and standard deviation were calculated from two biological replicates. An analogous experiment with wild-type Smc ATPase in shown is Figure S6C.
(C) Double insertion of peptide sequences (in red color) interferes with Smc function. Schematic view (left). Colony formation of strains harboring wild-type Smc, a Smc in-frame deletion (“Δ”), or a Smc with peptide insertions (“204Ω/996Ω”) at position 204 (residues SGPGGGGGRQVEP) and at position 996 (residues SGPGGGGGRQFER) on nutrient-poor (SMG) and nutrient-rich (ONA) medium.
(D) Cellular expression of Smc proteins harboring modified coiled coils. Smc-HaloTag proteins were labeled in crude Bs cell extracts by HaloTag-Oregon green and analyzed by in-gel fluorescence. To control for protein extraction, we stained an equivalent protein gel with Coomassie (bottom). “EQ” denotes the Smc(E1118Q) ATP hydrolysis mutation. “Mini-Smc” indicates a non-functional Smc variant with shortened coiled coil (CC293; Bürmann et al., 2017).
(E) In vivo cysteine cross-linking of Smc proteins harboring peptide insertions. Cross-linking of A715C and K1151C residues in Smc-HaloTag proteins bearing peptide insertions at positions 204 and 996. Data generation and display as in Figure 5D.
(F) Chromosome localization of Smc proteins with modified coiled coils. ChIP was performed with an antiserum raised against Bs Smc. Selected genomic positions were analyzed by quantitative PCR. The amount of ChIP DNA is given as percentage of input. The mean was calculated from three biological replicates. Data from individual experiments are displayed as dots with white, light gray, and dark gray filling, respectively. The same strains are also used in (D) and (E) and harbor a C-terminal HaloTag and the K1151C mutation. Equivalent results were obtained with a set of strains with untagged Smc and lacking additional Smc mutations (Figure S6D).
See also Figure S6.