Figure 2.
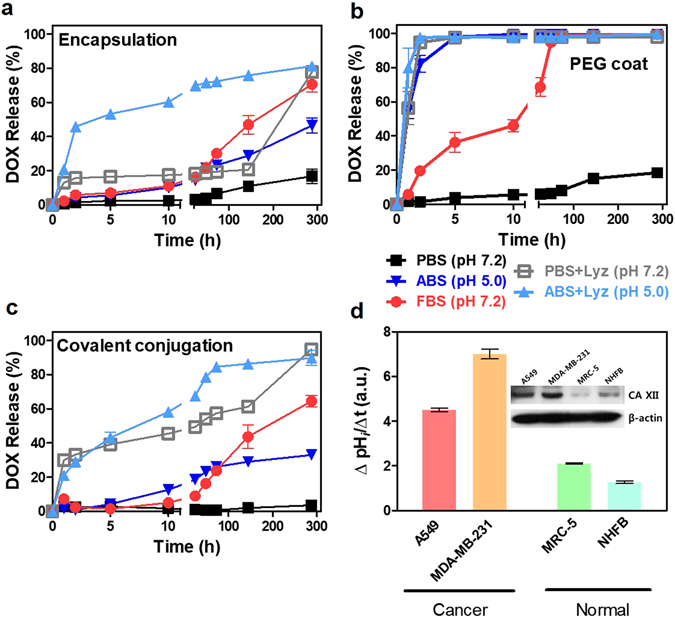
Extracellular and intracellular drug release analysis. (a–c) Released DOX by the different types of nanodrugs (i.e., covalent conjugation, liposomal encapsulation and PEG coat). (a) DOX release from encapsulation, (b) PEG coat and (c) covalent conjugation were analyzed in neutral (i.e., PBS), acidic (i.e., ABS), FBS (i.e., pH 7) and lysozyme-supplemented (i.e., both at pH 7 and pH 5) buffers up to 288 hrs. All data represent the mean ± SEM (n = 3). Most of the nanodrugs exhibited high drug stability in neutral PBS (pH 7.2) buffer. PEG coated drugs abruptly released (nearly 100% of the drugs), which detached from nanotubes in acidic buffer (pH 5.0 of ABS). In contrast, both covalent conjugation and liposomal encapsulation exhibited relatively stable drug release (i.e., less than 50% until 288 hrs) compared to PEG coat. PEG coated nanodrugs exhibited fast release (over 50% of the drugs were released within 10 hrs) in FBS buffer (10%, pH 7.2). (d) Western blot analysis for CA XII expression and measurements of acidification rate based on intracellular pH (pHi). Enhanced expression of CA XII proteins in A549 and MDA-MB-231 increased CO2/HCO3 − transporting machinery and, thus, facilitated intracellular acidification rate and provided more acidic intracellular conditions.
