Abstract
Although transcript levels have been traditionally used as a surrogate measure of gene expression, it is increasingly recognized that the latter is extensively and dynamically modulated at the level of translation (messenger RNA to protein). Over the recent years, significant progress has been made in dissecting the complex posttranscriptional mechanisms that regulate gene expression. This advancement in knowledge came hand in hand with the progress made in the methodologies to study translation both at gene-specific as well as global genomic level. The majority of translational control is exerted at the level of initiation; nonetheless, protein synthesis can be modulated at the level of translation elongation, termination, and recycling. Sequence and structural elements and epitranscriptomic modifications of individual transcripts allow for dynamic gene-specific modulation of translation. Cancer cells usurp the regulatory mechanisms that govern translation to carry out translational programs that lead to the phenotypic hallmarks of cancer. Translation is a critical nexus in neoplastic transformation. Multiple oncogenes and signaling pathways that are activated, upregulated, or mutated in cancer converge on translation and their transformative impact “bottlenecks” at the level of translation. Moreover, this translational dysregulation allows cancer cells to adapt to a diverse array of stresses associated with a hostile microenviroment and antitumor therapies. All elements involved in the process of translation, from the transcriptional template, the components of the translational machinery, to the proteins that interact with the transcriptome, have been found to be qualitatively and/or quantitatively perturbed in cancer. This review discusses the regulatory mechanisms that govern translation in normal cells and how translation becomes dysregulated in cancer leading to the phenotypic hallmarks of malignancy. We also discuss how dysregulated mediators or components of translation can be utilized as biomarkers with potential diagnostic, prognostic, or predictive significance. Such biomarkers have the potential advantage of uniform applicability in the face of inherent tumor heterogeneity and deoxyribonucleic acid instability. As translation becomes increasingly recognized as a process gone awry in cancer and agents are developed to target it, the utility and significance of these potential biomarkers is expected to increase.
Keywords: cancer, messenger RNA translation, dysregulation, biomarker, biological tumor marker, eIF4E, translational machinery, neoplastic transformation
Introduction
Although our understanding of transcriptional regulation and dysregulation in cancer has significantly advanced over the last decade, comparatively little is known about dysregulation of gene expression at the translational level. However, it is recognized that gene expression is extensively and dynamically modulated at the translational level accounting to a large extent for the discrepancies between messenger RNA (mRNA) and matching protein levels. Although transcript levels have been traditionally used as a proxy of the protein abundance in a cell, genomic-scale studies have shown that the latter is predominantly controlled at the posttranscriptional level and especially translation (1). Translation represents a more proximal level of control, allowing the cell to adapt swiftly to stress conditions by modulating protein synthesis from an existing pool of mRNAs, unlike the process of transcription which mediates more permanent changes in cell physiology or fate (2). Indeed, studies have shown that in response to stress, changes in translation precede and are of a greater magnitude than changes in transcription (3). In fact, immediate changes in the translation of transcripts encoding for transcription factors steer the later-appearing and more permanent changes in transcription (3). Translation and translational control is emerging as a critical nexus in mediating adaptive stress responses allowing cancer cells to overcome a diverse array of stress conditions imposed on them by the tumor microenvironment, immune recognition, their own continuous replication, and therapeutic modalities. Simultaneously, qualitative and quantitative changes in the translational machinery are critical mediators that carry out the transformative impact of oncogenes and oncogenic signaling.
This review provides an overview of translation and the regulatory mechanisms that govern it in normal cells. We discuss ways whereby translation becomes dysregulated in cancer and how oncogenic insults uniformly converge at the level of translation and modulate the translational landscape. Indeed all factors involved in carrying out the process of translation, from the mRNA which provides the template of the protein to be synthesized, the components of the translational machinery tRNA and ribosomes, to the multiple and diverse proteins that interact with the mRNA, can be perturbed in cancer. Coordinate changes in any or all of these elements may contribute significantly to the malignant behaviors of the transformed cells. In this context, mediators or components of translation that are aberrantly expressed or modified in cancer arise as biomarkers with potential prognostic or predictive significance (Table 1; Table S1 in Supplementary Material). Although none of these factors has reached broad clinical applications, extensive preliminary work has correlated aberrations in the components of translation with clinical outcomes. Since translation is a downstream process into which diverse arrays of oncogenic pathways converge, one potential advantage of these biomarkers is their fairly uniform perturbation, even in the face of tumor heterogeneity underpinned by genomic instability and multiple, redundant, interwoven, and bypassing signaling cascades. This advantage has been exemplified in BRAF(V600)-mutant melanoma, colon, and thyroid carcinoma, whereby the formation of eIF4F translation initiation complexes was the common point of convergence of multiple pathways that conferred resistance to targeted anti-BRAF, anti-MEK, and anti-BRAF plus anti-MEK drug combinations (4).
Table 1.
Common malignancies in which the utility of factors or regulators of translation has been explored as potential biomarkers.
| Organ of origin | Factor |
|---|---|
| Breast | hnRNP A1 (5), Y-box binding protein 1 (YB-1) (6), HuR (7, 8), IGF2BP3 (9), eIF4E (10–15), 4E-BP1 (16), eIF4AI (12), eIF4B (12), eIF4G (17), PCDC4 (12), fibrillarin (FBL) (18), elongator acetyltransferase complex subunit 3 (ELP3) and CTU1/2 (19) |
| Lung | YB-1 (20), HuR (21, 22), PCDC4 (23), tRNA isopentenyltransferase 1 (TRIT1) (24) |
| Gastrointestinal tract | hnRNP A1 (25), YB-1 (26–28), HuR (29–31), IGF2BP3 (32–35), eIF4E (36, 37), 4E-BP1 (38), PCDC4 (39–41) |
| Prostate | hnRNP C (42), YB-1 (43), eIF4E (44) |
| Gynecologic malignancies | YB-1 (45), HuR (46, 47), IGF2BP1 (48), IGF2BP3 (49, 50), 4E-BP1 (51), PCDC4 (52) |
| Central nervous system | YB-1 (53), IGF2BP1 (54), PCDC4 (55), eEF2 kinase (eEF2K) (56), TRM6/61 (57) |
Overview of the Eukaryotic Translation
The process of translation can be divided into four major phases: initiation, elongation, termination, and ribosome recycling.
During the initiation phase, a preassembled 43S preinitiation complex is recruited to the m7Gppp capped 5′ end of the mRNA (2). The 43S preinitiation complex is formed by the association of the 40S ribosomal subunit with the eukaryotic initiation factors (eIF) 1, 1A, 2, 3, 5, and the ternary complex (composed of eIF2, initiator Met-tRNAi, and GTP). Preinitiation complexes attach to 5′ capped untranslated region (UTR) with the cooperative action of eIF4F and eIF4B (58).
eIF4F is composed of the DEAD-box RNA helicase eIF4A, the 5′ cap-binding protein eIF4E, and the scaffolding protein eIF4G (58). eIF4A in mammals exists two highly related isoforms [eIF4AI and eIF4AII; a third isoform, eIF4AIII, acts as a translation initiation factor specifically for the nuclear cap-binding complex bound mRNAs and efficiently unwinds secondary structures in their respective 5′ UTRs (59)] which, despite being interchangeable in the eIF4F complex and sharing 90% homology (60), they seem to have some important functional distinctions (61). The activity of eIF4A is modulated by two homologous RNA-binding proteins, eIF4B and eIF4H (60, 62). eIF4B and eIF4H stimulate the helicase activity of eIF4A, allowing the latter to unwind longer and more stable 5′ UTR structures (63). They also stabilize single-stranded unwound 5′ UTR regions and prevent reannealing. In doing so, they also promote unidirectional 5′ to 3′ ribosome scanning (62). eIF4B and eIF4H share a common binding site on eIF4A and, accordingly, their interactions with eIF4A are mutually exclusive (64). Furthermore, eIF4F-bound eIF4E stimulates the helicase activity of eIF4A independent of eIF4E’s 5′ cap-binding function (65). Last, eIF4G also interacts with the poly(A)-binding protein (PABP); PABP associates with the mRNA 3′ poly(A) tail to circularize and stabilize the mRNA (66). The eIF4G–PABP interaction is not absolutely required for ribosome recruitment but enhances translational efficiency (67).
Besides eIF4E and eIF4F, other cap-binding proteins and complexes have been described (68, 69) that drive selective translation. eIF4E2 is a cap-binding homolog of eIF4E which mediates the selective translation of transcripts involved in the adaptive hypoxic response (69). Under hypoxic conditions, eIF4E is sequestered by hypophosphorylated 4E binding protein (4E-BP) (see Regulation of eIF4F Complex Assembly and Activity) while eIF4E2 is relatively spared; the RNA-binding protein RBM4 recruits hypoxia-inducible factor 2α (HIF2α) to a specific RNA element (RNA hypoxia response element) in the 3′ UTR of select mRNAs and this complex in turn diverts translation of the respective RNAs through the cap-bound eIF4E2 (69). eIF4E2-mediated translation has been shown to be critical for tumor growth in sizes greater than the oxygen diffusion limit (70). Oxygen tension as well as development and differentiation status seems to determine the switch between eIF4E2- and eIF4E-mediated modes of cap-dependent translation initiation (71). Last, eIF3-specialized translation is a recently described mode of translation initiation that involves 5′ cap recognition by eIF3d (68, 72). An internal stem–loop structure in the 5′ UTR is also concurrently required for eIF3 recognition and recruitment (68, 72). The genes that are amenable to this mode of translation have been identified using photoactivatable ribonucleoside-enhanced crosslinking and immunoprecipitation and gene ontology analysis revealed that they are functionally enriched in cancer-associated cell growth regulatory pathways, including apoptosis, cell cycling, and differentiation (72). Examples of these genes include c-Jun, calcineurin B, CDK12, and BTG1. Interestingly, eIF3 has opposing translational regulatory functions: it promotes the translation of c-JUN, which is a proto-oncogene; while it blocks the translation of BTG1, whose overexpression impairs invasive growth in human lung cancer cells (72). The factors that determine the transcript-specific translational modulatory function of eIF3 is an open question. Intriguingly, a cis-acting RNA element has been identified in the c-Jun 5′ UTR that blocks eIF4F even when the 5′ cap is intact and the internal stem-loop eIF3-recognition site is deleted (68). Such elements that block or render the association of the eIF4F with the 5′ cap inefficient are being increasingly recognized and their role is to direct mRNAs into a specific translation pathway (72) [see below, translation inhibitory elements (TIEs)].
The preinitiation complex scans downstream the 5′ UTR, inspecting successive triplets as they enter the P(peptidyl) decoding site of the ribosome for complementarity to the anticodon of Met-tRNAi (2). Hydrolysis of eIF2-bound GTP is stimulated by eIF5 in the scanning preinitiation complex, but completion of the reaction is impeded at non-AUG triplets (73). A perfect match with an AUG start codon triggers the arrest of scanning and the irreversible hydrolysis of the GTP in the ternary complex (2). Start codon recognition leads to the release of the initiation factors and the joining of the large (60S) ribosomal subunit to form the 80S initiation complex. The 80S complex is then ready to accept the appropriate aminoacyl-tRNA into the A (aminoacyl) site and synthesize the first peptide bond (2).
Translation of some eukaryotic mRNAs can be initiated independently of the m7Gppp cap by recruitment of the 40S ribosomal subunit to a cis-acting element located in the 5′ UTR called internal ribosome entry site (IRES). IRES activity is modulated (usually enhanced) by proteins called IRES trans-acting factors (ITAFs) (74). IRESs are structurally and functionally diverse (75). There is no uniformity in the way they operate as well as their factor requirements. Variable interactions with canonical initiation factors, ITAFs, and the 40S ribosome are thought to lead to proper positioning of the initiation codon to the ribosomal P-site (76). For some IRESs, the recruitment of the 40 S ribosome involves mRNA–ribosome RNA (rRNA) base pairing between the IRES and 18S rRNA (77, 78). In addition, TIEs in the 5′ UTR may block cap-dependent translation and divert the translation of the respective transcripts through the IRES (79). rRNA methylation and pseudouridylation enhance IRES-mediated translation (18, 80, 81); these covalent modifications are mediated by the enzymes fibrillarin (FBL) (18) and dyskerin (DKC1) (81), respectively. Also, ribosomal proteins ribosomal protein L38 (79) and ribosomal protein S25 (82) have been shown to be important for IRES-mediated translation. These covalent modifications and ribosomal protein associations modulate the mRNA affinities and IRES-translational capabilities of the ribosomes. IRES-mediated translation is frequently upregulated during stress conditions when cap-dependent translation initiation is compromised (83). However, for some transcripts containing a TIE such as the Hox mRNAs, IRES-mediated translation may constitute the only mode of translation initiation (79). An increasing number of genes with unequivocal relevance to cancer biology have been found to contain IRES; examples include VEGF (84), Bcl-2 (85), FGF1 (86), c-Jun (87), Aurora A kinase (88), c-myc (89, 90), XIAP, and IGF1R (78, 91). To that end, the expression of ITAFs that modulate the translation of these mRNAs has been investigated and correlated with clinicopathologic parameters and outcomes and may serve as a biomarker in specific cancers (Table 1; Table S1 in Supplementary Material). Although studies have shown that typically IRES-mediated translation is upregulated under cancer-relevant stress conditions, the overall role of this non-canonical mode of translation initiation seems to be more complex, as transcripts encoding proapoptotic proteins such as the apoptotic protease activating factor (APAF1) also contain IRESs (92). Changes in the abundance or activity of the ITAFs (83) and alterations in the composition of specific ribosome subpopulations may modulate IRES-mediated translation.
Translation elongation is mediated by elongation factors eEF1 and eEF2 (93). eEF1A in complex with GTP binds to and delivers aminoacylated tRNA to the A-site of the ribosome. GTP is hydrolyzed when codon-anticodon recognition occurs; the eEF1A-guanosine-5’-diphosphate (GDP) then exits the ribosome and is recycled to eEF1A-GTP by the nucleotide exchange factor eEF1B complex (94). eEF2 mediates the translocation of the nascent protein chain from the A-site to the P-site of the ribosome. eEF2 can be phosphorylated and inactivated by its cognate kinase eEF2 kinase (eEF2K), resulting in deceleration of translation elongation (95). eEF2K in turn is regulated by a diverse array of inputs including the mTORcomplex 1 (mTORC1) pathway among other nutrient-sensing and growth factor activated signaling pathways. Ribosome profiling has shown that global translation elongation rates are remarkably consistent across diverse classes of transcripts (96). However, at a single mRNA level, ribosomes move in a stop-and-go manner and can pause at various consensus sites which, remarkably, do not correspond to rare codons where tRNA recruitment might be expected to be rate limiting (96).
Translation termination in eukaryotes occurs in response to stop codons in the ribosomal A-site and it involves the concerted action of two eukaryotic release factors eRF1 and eRF3 (97, 98). eRF1 is responsible for stop codon recognition and the hydrolysis of peptidyl-tRNA, whereas eRF3 strongly stimulates peptide release by eRF1 in a GTP-dependent manner. After peptide release, eRF1 remains bound to post-termination complexes and, together with the ATP-binding cassette protein ABCE1, dissociates the complex into a 60S subunit and tRNA- and mRNA-associated 40S subunits (99). ABCE1also mediates the recycling of post-termination complexes (100), an essential process in maintaining a pool of free ribosomes in the cell.
In some cases, post-termination complexes do not undergo complete recycling; 40S subunits remain bound to mRNA, and termination is followed by reinitiation, usually downstream of the stop codon (98). Indeed, multiple studies that employed ribosome profiling have revealed translation in the 3′ UTRs (100, 101) originating from reinitiation rather than readthrough of the main open reading frame (ORF) stop codon (100). 3′ UTR translation is upregulated under conditions of nutrient starvation in the yeast (100); other stress conditions that upregulate this pattern of translation and its implications in cancer are an open question. The overwhelming majority of such reinitiation events seems to occur when the 5’-proximal ORF is short and can significantly impact the translation of the downstream protein-coding ORF (98). This is particularly relevant in the human transcriptome where nearly half of all transcripts are at least computationally predicted to contain upstream or overlapping reading frames whose translation in general represses the translation of the downstream coding sequence (102). It is generally thought that reinitiation involves the same factors as standard initiation; however the complex MCT-1/DENR is a non-canonical initiation factor that specifically promotes reinitiation in eukaryotes (103). In fact, certain mRNAs containing upstream ORFs (uORFs) with strong Kozak sequences (i.e., consensus sequences that promote translation) selectively require MCT-1/DENR for the proper translation of the downstream main coding sequence, and interestingly, these mRNAs are enriched for transcriptional regulators and oncogenic kinases (103).
Translational Regulation and Dysregulation in Cancer
Transcript-Specific Elements That Modulate Translation
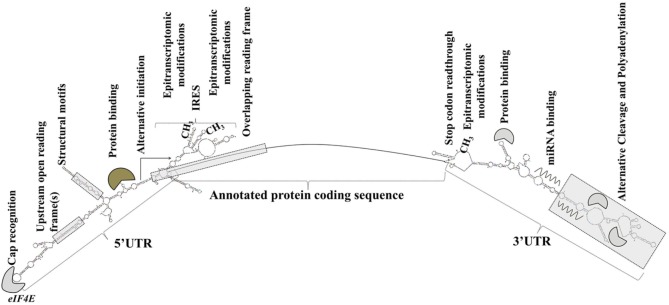
Sequence elements and structural features of the individual mRNA transcript allow for the dynamic modulation of its translation (93) (Figure 1).
Figure 1.
Transcript-specific elements that modulate messenger RNA metabolism, translation initiation, and efficiency. Gene expression is extensively and dynamically modulated at the post-trascriptional level in cell type-specific and context-dependent manner.
Elements in the 5′ UTR include cap recognition patterns (discussed below in relation to eIF4E, the principle cap-binding protein), IRESs, uORFs, motifs with RNA-binding protein recognition sequences or specialized secondary structures, and epitranscriptomic modifications. An example of a 5′ UTR structural element with a transcript-specific translational impact is a 12-nucleotide guanine quartet (CGG)4 motif that can form RNA G-quadruplex structures (104). The presence of RNA G-quadruplexes renders the translation of the respective transcripts remarkably sensitive to eIF4A inhibition with the investigational compound silvestrol (104). Transcripts of many oncogenes, superenhancer-associated transcription factors, and epigenetic regulators are eIF4A-dependent and, accordingly, silvestrol-sensitive (104).
3′ UTRs are typically longer than the 5′ UTRs and are thought to allow for greater transcript-selective translational regulation (105). The 3′ UTRs undergo epitranscriptomic modifications and contain miRNA-binding sites; the latter silence gene expression by translational repression and mRNA destabilization (106, 107). Studies have shown that the 3′ UTR constitutes the site where most mRNA–RNA-binding protein interactions occur (108). Through a process called alternative cleavage and polyadenylation (109), cells can modulate the length of the 3′ UTR and consequently, by retaining or excluding miRNA- or RNA protein binding sites, regulate the function, stability, localization and translation efficiency of the respective mRNAs. Cancer cells usurp this mechanism to promote the silencing of antiproliferative genes and evade growth inhibition (109, 110).
RNA-Binding Proteins
RNA-binding proteins impact all aspects of RNA biology including transcription, pre-mRNA splicing, polyadenylation, RNA modification, transport, localization, translation, and turnover. Although they bind to RNA through a relatively small repertoire of RNA-binding scaffolds, their affinity and specificities are extensively modulated by auxiliary domains which in turn modulate interactions with other proteins and are subject to posttranslational modifications (111). Although traditionally thought that RNA-binding proteins bind to the 5′ and 3′ UTRs of their target transcripts, the former can also bind, probably to a lesser extent, to the protein-coding region of the message. Depending on the binding site across the target mRNA, RNA-binding proteins can mediate different functions. This has been exemplified with GLD-1 whereby binding to sites across the protein-coding regions of its target transcripts mediated predominantly translational repression (112). RNA-binding proteins with relevance to cancer biology include HuR, the IGF2 mRNA-binding protein (IGF2BP) family (mainly IGF2BP1 and IGFBP3), and cytoplasmic polyadenylation element-binding (CPEB) (113).
HuR (ELAV-like RNA-binding protein 1) stabilizes and/or affects the translation of its target mRNAs by interacting with one or several U- or AU-rich elements (AREs) in their 3′ UTRs (114). Other functions attributed to HuR include involvement in pre-mRNA splicing (115–117) and nuclear export of mRNAs (114). HuR’s function is predominantly regulated by posttranslational modifications which in turn determine HuR’s interactions with its target mRNAs and nucleocytoplasmic shuttling machinery (114). A number of cancer-related transcripts, including mRNAs for proto-oncogenes, cytokines, growth factors, and invasion factors, contain AREs and have been identified as HuR targets. Examples include c-myc, IGF1R, HIF1α, HSP90AB1, eEF2, interleukin 11, and CDK6 (116). It has been proposed that HuR has a central tumorigenic activity by enabling multiple cancer phenotypes (118). HuR localizes predominantly in the nucleus where it interacts with introns and is involved in pre-mRNA splicing (115–117). HuR’s cytoplasmic translocation is thought to be the initial and critical step for its target mRNA stabilizing and translational modulatory effects (114, 119, 120). Multiple studies have consistently associated cytoplasmic (but not nuclear) expression with worse clinicopathologic characteristics and outcomes in diverse malignancies (Table 1; Table S1 in Supplementary Material).
The IGF2BPs are oncofetal proteins that are normally expressed only during embryogenesis (121); however, the expression of IGF2BP1 and IGF2BP3 is induced in various malignancies. Examples include neuroblastoma (54) and ovarian carcinoma (48) for IGF2BP1; and triple-negative breast cancer (9), ovarian (49) and endometrial (50) clear cell carcinoma, gastric adenocarcinoma (32), cholangiocarcinoma (33, 122), colon cancer (34, 35), renal cell (123, 124), and urothelial carcinoma (125) for IGF2BP3 (Table 1; Table S1 in Supplementary Material). During development, IGF2BPs are required for proper nerve cell migration and morphological development, presumably by regulating cytoskeletal remodeling and dynamics (126). Likewise, in tumor cells, IGF2BPs modulate cell polarization, adhesion, and migration. Moreover, they are highly associated with cancer metastasis and the expression of oncogenic factors (KRAS proto-oncogene GTPase, MYC, and MDR1) (126). In fact, IGF2BP1 was originally identified as a protein involved in the stabilization of c-myc mRNA (127). Furthermore, IGF2BP1 is an ITAF upregulating the IRES-mediated translation of the cellular inhibitor of apoptosis 1 leading to resistance to apoptosis in rhabdomyosarcoma (128). Multiple studies have evaluated the expression of IGF2BP proteins (mainly IGF2BP3) in cancer and have consistently correlated IGF2BP3 expression with the subsequent development of recurrence or metastases in localized cancer and worse clinical outcomes (Table 1; Table S1 in Supplementary Material). There is significant homology among the 3 IGF2BP family members, thus antibodies used to investigate the expression of IGF2BP1 and IGF2BP3 may have not differentiated the paralogs (129).
Last, the CPEB family of RNA-binding proteins (CPEB1–4) bind to cytoplasmic polyadenylation elements (CPEs; consensus sequence UUUUUAU) in the 3′ UTR of target mRNAs (113, 130) and modulate translation (both activation and repression) through regulation of poly(A) tail length (131). Multiple lines of evidence suggest a tumor-suppressive role for CPEB1 (130). The expression level of CPEB1 mRNA are decreased in a diverse array of malignancies and this reduction has been associated with proliferation, invasion, angiogenesis, increased resistance to nutritional stress, loss of polarity, and epithelial-to-mesenchymal transition (130, 132, 133). CPEB4 on the other hand is overexpressed in pancreatic ductal adenocarcinoma, high-grade gliomas (134), and early in the development of melanoma (135). CPEB4 associates with a large number of CPE-containing mRNAs, which seem to be tissue-specific (134, 135), and steers the translational landscape to support the phenotypic hallmarks of malignancy, i.e., invasive growth (pancreatic adenocarcinoma), uncontrolled tumor growth, and aberrant angiogenesis (high-grade gliomas) (134).
Epitranscriptomic Modifications
Although RNA modifications have been known for nearly 60 years, only recently, has it been appreciated how extensive and dynamic these chemical modifications to the mRNA may be (136). Recent technical advances have led to the discovery, identification, and mapping of widespread mRNA modifications with N6-methyladenosine (m6A), 5-methylcytosine (m5C), pseudouridine (ψ), N6,2’-O-dimethyladenosine (m6Am), 5-hydroxylmethylcytosine (hm5C), inosine (I), and N1-methyladenosine (m1A) (137, 138). Analogous to the epigenetic modifications of the deoxyribonucleic acid (DNA), mRNA modifications involve epitranscriptomic “writers,” “erasers,” and “readers”; i.e., enzymes that insert, remove, or recognize these covalent modifications, respectively, and facilitate mRNA-templated processes. Single nucleotide polymorphisms in the m6A eraser alpha-ketoglutarate dependent dioxygenase (FTO), a gene that traditionally has been associated with obesity, have been associated with melanoma (139) and estrogen receptor-negative breast cancer (140). These mRNA modifications can potentially affect most posttranscriptional steps in gene expression and, specifically for m6A, are highly dynamic (137). Common themes that arise so far link m6A modifications with mRNA stability, splicing, and translational efficiency (137). m6A sites are enriched near stop codons (141) and an association between m6A modification and proximal alternative cleavage and polyadenylation, i.e., 3′ UTR shortening has been established (142). m6A modifications in the 5′ UTR have been associated with cap-independent translation (143, 144). The role of the epitranscriptome in cancer remains an open area of investigation.
RNA Editing
The most frequent type of RNA editing in the human transcriptome is adenosine-to-inosine (A-to-I), which involves hydrolytic deamination of adenosine by the adenosine deaminase, RNA-specific (ADAR) family of RNA editases, ADAR1, ADAR2/ADARB1, and ADAR3/ADARB2 (145). The resulting inosine bases are subsequently read as guanosines, thus inducing A-to-G posttranscriptional changes. Most RNA editing sites occur in non-coding sequences, such as 5′ UTRs, 3′ UTRs, and intronic sequences (145). ADARs may influence gene expression by modulating mature miRNA biogenesis, splicing, alternative cleavage, and polyadenylation (146). Aberrant activation of ADAR-mediated RNA editing has emerged as a driver of cancer progression (145) but how it distorts the translational landscape is an open question.
Regulation of eIF4F Complex Assembly and Activity
Oncogenes and oncogenic signaling pathways uniformly converge at the level of translation and the transformative potential of oncogenic insults “bottlenecks” at the level of translation (44, 147, 148): ribosomal protein (147) and eIF4E haploinsufficiency (148) or non-phosphorylatable eIF4E (44) suppress the transformative potential of Myc and Ras oncogenes.
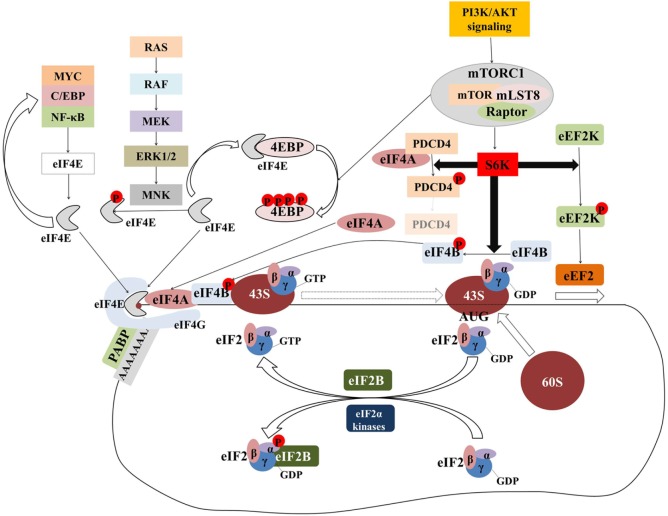
Initiation has been considered the rate-limiting step in translation and has been intensively investigated. Figure 2 provides an overview of the components and regulation of translation initiation. In many human cancers, eIFs are either overexpressed or ectopically activated by oncogenic signaling cascades, resulting in increased survival and accelerated proliferation (149). To that end, many eIFs are considered bona fide proto-oncogenes (149). Multiple studies in diverse malignancies and settings have investigated correlations between expression levels of eIFs with clinical parameters or outcomes (Table 1; Table S1 in Supplementary Material). Components of the eIF4F complex are perhaps the most intensively investigated factors with potential utility as biomarkers. In the sections that follow, we outline the pathobiology of eIF4F and its components which underpins its potential utility as a biomarker.
Figure 2.
The majority of translational regulation is exerted at the level of initiation. Overview of the regulation of eIF4F and ternary complex assembly and activity. Transcription factors (MYC, C/EBPα, NF-κB) upregulate the transcription of components of the eIF4F complex which in turn upregulates the translation of transcripts encoding for the former generating a “feed-forward” loop. Signaling pathways activate the MNK serine/threonine kinases which in turn phosphorylate eIF4E. Although eIF4E phosphorylation is associated with the acquisition of critical hallmarks of malignancy, the mechanism by which this is achieved is an open question. The mTORcomplex 1 (mTORC1) is a critical nexus linking aberrant signaling with translation. Phosphorylation of the 4E binding proteins (4E-BPs) releases eIF4E to form eIF4F complex. Activation of ribosomal protein S6 kinases (S6Ks) leads to the phosphorylation and subsequent degradation of programmed cell death 4 (PDCD4), which in turn releases eIF4A to form eIF4F complex. At the same time, phosphorylated eIF4B stimulates the helicase activity of eIF4A which is necessary to unwind the highly structured 5′ untranslated regions of many oncogenes. Phosphorylation of eEF2 kinase (eEF2K) leads to its inactivation and accelerated translation elongation by the uninhibited eEF2. Ternary complex formation is regulated by means of eIF2α phosphorylation. eIF2B catalyzes the guanosine-5′-diphosphate (GDP)-to-GTP exchange to allow another cycle of translation initiation to occur. In response to stress, eIF2α is phosphorylated at serine 51 and becomes a competitive inhibitor of eIF2B. Poly(A)-binding protein (PABP) associates with the messenger RNA (mRNA) 3′ poly(A) tail to circularize and stabilize the mRNA and bolster its translation.
The Oncogenic Transformative Potential of eIF4E
eIF4E was the first translation initiation factor documented to have oncogenic transformative potential (150, 151). Although eIF4E is the least abundant (i.e., most limiting) of the initiation factors (60), multiple studies have shown that partial depletion of eIF4E has only a moderate impact on protein synthesis rates (152). Indeed, mice haploinsufficient in Eif4e develop normally with near normal global mRNA translation levels (148). This Eif4e excess becomes critical in the context of oncogenic signaling (148). Although eIF4E is required for cap-dependent translation of all mRNAs, changes in its levels have a highly selective (as opposed to global) impact on a subset of mRNAs that encode proteins with prosurvival and proliferative functions. Transformed cells usurp this excess Eif4e to translate genes involved in cell signaling, apoptosis, ribosome biogenesis, control of proteasome activity, nucleotide biosynthesis, oxidative phosphorylation, and the oxidative stress response, all of which act in concert to promote tumorigenesis (148). Many of these eIF4E-responsive mRNAs possess complex and highly structured 5′ UTRs (153) that impede ribosome recruitment and scanning; eIF4E availability allows the cell to overcome this impediment by virtue of stimulating eIF4A helicase activity (65). The basis of eIF4E translational selectivity may also rely on the fact that, although m7Gppp is the invariant component of the 5′ cap, there is variability in the affinity with which eIF4E associates with the 5′ cap determined by the second nucleotide (67, 154). Of note, the first and second nucleotide following the m7Gppp can be variably methylated; the physiologic significance of these modifications is not yet known. Moreover, the association of the eIF4E with the cap blocks decapping leading to mRNA stabilization (67). Besides its role in translation, eIF4E mediates nucleocytoplasmic transport of specific transcripts such as cyclin D1 which promotes cell cycle progression, and this function contributes to the transformative activity of eIF4E (155).
A similarly selective impact on translation has been observed with eIF4E phosphorylation. eIF4E is phosphorylated by the MNK1/2 serine/threonine kinases, which are activated in response to mitogenic and stress signaling downstream of ERK1/2 and p38 MAP kinase, respectively (Figure 2). eIF4E phosphorylation [which is dispensable for normal growth and development and, per se, does not globally affect protein synthesis (156)] is associated with upregulated translation of a select subset of protumorigenic mRNAs (44). The mechanistic basis by which phosphorylated eIF4E achieves that remains a critical unanswered question; in fact, in a seemingly counterintuitive fashion, phosphorylated eIF4E has lower affinity for the cap structure (60, 157, 158). Nonetheless, eIF4E phosphorylation underpins epithelial-to-mesenchymal transition, invasion, and migration (159, 160) and is associated with higher-grade and hormone-refractory prostate cancer (44).
Signaling Pathways Upregulate eIF4F Assembly and Activity through Mechanistic or Mammalian Target of Rapamycin (mTOR)
The mammalian/mechanistic target of rapamycin is a serine/threonine kinase that forms 2 distinct multiprotein complexes termed mTOR complex 1 and 2 (Figure 2). Each complex has distinct substrates and, accordingly, regulates different cellular processes (161). mTORC1 links many extracellular and intracellular nutrient and growth cues to the translation process mainly by regulating the eIF4F assembly (66). Simultaneously, mTORC1 (in association with CK2) regulates ternary complex recycling to couple coordinate changes in eIF4F assembly and, consequently modulate the rate-limiting step of translation initiation (162).
mTORcomplex 1 stimulates global protein synthesis, as well as translation of a specific subset of mRNAs (163). Eukaryotic translation initiation factor 4E-BPs and ribosomal protein S6 kinases (S6Ks) are the most extensively studied and best-understood downstream effectors of mTORC1 (163).
4E binding proteins modulate eIF4F assembly by competing with eIF4G for the same binding site on eIF4E. 4E-BPs’ affinity for eIF4E depends on the phosphorylation status of the former, which in turn depends on the activity of mTORC1. Activation of the mTORC1 pathway leads to the phosphorylation of 4E-BPs, which in turn dissociate from eIF4E allowing the latter to associate with eIF4G and form the eIF4F complex. In mammals, there are three known 4E-BPs (4E-BP1, 4E-BP2, and 4E-BP3). The activity of 4E-BP1 and 4E-BP2 is primarily controlled by phosphorylation. mTOR inhibitors downregulate 4E-BP1 and 4E-BP2 phosphorylation leading to eIF4E sequestration. However, a common mechanism of acquired resistance to mTOR inhibitors involves downregulation of 4E-BP1 and 4E-BP2, which leads to increased eIF4E availability. The eIF4E:4E-BP ratio has been proposed as a predictive biomarker of response to mTOR inhibitors that can personalize treatment selection (164). Unlike 4E-BP1 and 4E-BP2, 4E-BP3 is mainly regulated by transcriptional induction (165). 4E-BP3 is an important determinant in mediating the antiproliferative effects of mTOR inhibitors and induction of its expression has been associated with antitumor response to mTOR inhibition (165). 4E-BP3 induction of expression upon mTOR inhibition can potentially predict response as well as duration of response associated with mTOR inhibition.
Ribosomal protein S6 kinases (S6K1 and S6K2 in mammals) have various downstream substrates including ribosomal protein S6, eIF4B, and programmed cell death 4 (PDCD4). The impact of ribosomal protein S6 phosphorylation on translation is not very well understood. eIF4B, however, is an important auxiliary factor that stimulates the helicase activity of eIF4A; its phosphorylation by S6K (166) as well as other kinases (167) selectively and positively impacts the translation of protumorigenic transcripts with structured 5′ UTRs (168). The phosphorylation of PDCD4 by S6Ks leads to its proteasomal degradation and the liberation of eIF4A from PDCD4-eIF4A inhibitory complexes (169). Alongside upregulating translation initiation, S6Ks also phosphorylate eEF2K which in turn, accelerates elongation. Last, mTORC1 upregulates ribosome biogenesis by activating RNA polymerase I transcription initiation factor TIF-1A (170), and tRNA synthesis by phosphorylating and suppressing the RNA polymerase III inhibitor MAF1 (171, 172) (see tRNA Abundance and Modifications Fostering Oncogenic Translation).
Oncogenic Transcription Factors Upregulate the eIF4F Complex
The c-myc oncogene has a pervasive impact on translation and the upregulation of translational output is a critical determinant of its oncogenic activity in vivo (147). Myc modulates the transcription of 10–15% of all genes and Myc-target genes belong to diverse functional categories (173); specifically regarding translation, Myc upregulates ribosome biogenesis, tRNA levels, and key translation initiation and elongation factors (174). All components of the eIF4F complex (eIF4E, eIF4AI, and eIF4GI) are under the direct transcriptional control of c-Myc and are coordinately upregulated when c-myc is overexpressed (174, 175). Increased eIF4F, in turn, selectively upregulates the translation of protumorigenic mRNAs including c-Myc mRNA (Figure 2). Normally, the activity of this c-Myc–eIF4F feed-forward loop is modulated by negative regulators of Myc or downregulators of eIF4F assembly [e.g., mTORC1, see Signaling Pathways Upregulate eIF4F Assembly and Activity through Mechanistic or Mammalian Target of Rapamycin (mTOR)]. In cancer, however, the c-Myc–eIF4F feed-forward loop fuels neoplastic progression as the negative checkpoints of this loop are circumvented by mutations or perturbations in signaling pathways (174).
Other transcription factors can also modulate eIF4F assembly and activity (66). In a positive feedback loop, the leukemogenic p30 isoform of C/EBPα upregulates the transcription of eIF4E and eIF4E in turn upregulates the translation of C/EBPα (176). The dysregulated activity of this loop may underpin the dysplastic phenotype of myelodysplastic syndrome/acute myelogenous leukemia (AML) associated with nucleophosmin deficiency (176). In the context of M4 and M5 AML, NF-κB directly upregulates the transcription of eIF4E; this association has not been seen in other AML subtypes or normal hematopoietic cells (177) suggesting that other transcription factors besides c-Myc may upregulate eIF4E in a context-dependent or tissue-specific manner. Under conditions of hypoxia in breast cancer cells, HIF1α promotes eIF4E1 expression acting through hypoxia response elements in the proximal promoter region of eIF4E1 (178). In the context of this adaptive response, eIF4E1 upregulates the translation of a select subset of mRNAs important for mammosphere formation and growth (178).
Posttranscriptional Upregulation of eIF4E in Cancer
The eIF4E mRNA stability is modulated by 2 competing 3′ UTR RNA-binding proteins, HuR (see RNA-Binding Proteins) and AU-rich binding factor 1 (AUF1/HNRNPD) (179). In cancer, overexpression of HuR leads to eIF4E mRNA stabilization and consequently elevated eIF4E protein levels. By increasing the stability of multiple client transcripts and through the upregulation of translation of a select subset of protumorigenic mRNAs, HuR and eIF4E, respectively, coordinately dysregulate gene expression at the posttranscriptional and translational level (179).
Regulation of Ternary Complex Formation
eIF2 consists of an α, β, and γ subunit and cycles between a GDP- and GTP-bound forms. Following start codon recognition, eIF2-bound GTP hydrolysis is completed resulting in the formation of eIF2–GDP complex, which in turn dissociates from the 40S ribosomal subunit (180). For another cycle of initiation to occur, GDP bound to eIF2 is replaced by GTP by the guanine exchange factor eIF2B (180). In response to virtually all stresses, eIF2α is phosphorylated at serine 51 resulting in the conversion of eIF2α from a substrate to a competitive inhibitor of eIF2B (180, 181) (Figure 2). Four eIF2α kinases catalyze this phosphorylation in a cell type- and stress-specific manner: protein kinase RNA-activated (PKR) is activated by viral infection, PKR-like endoplasmic reticulum kinase (PERK) is activated by the accumulation of unfolded polypeptides in the lumen of the endoplasmic reticulum, general control non-derepressible 2 kinase (GCN2) is activated by amino acid starvation and ultraviolet (UV) light, and heme-regulated eIF2α kinase (HRI) is activated by heme deficiency and redox stress. Since eIF2B is present in limiting concentrations, phosphorylation of even a small fraction of eIF2α significantly inhibits eIF2–GDP–eIF2–GTP recycling leading to a global inhibition of protein synthesis (181). Nonetheless, the phosphorylation of eIF2α enhances the translation of a select group of mRNAs, which encode for proteins involved in stress adaptation and recovery (181).
The mechanism by which the eIF2α phosphorylation status modulates translation of a given mRNA relies on the presence or absence of uORFs. This has been exemplified by the modulation of the expression of the transcription factor ATF4 (182). The mouse activating transcription factor 4 (ATF4) mRNA has two uORFs. When eIF2-GTP is abundant in non-stressed cells, ATF4 expression is downregulated as ribosomes scanning downstream of uORF1 reinitiate at the next coding region, uORF2, which overlaps with the ATF4-coding sequence. During stress conditions, phosphorylation of eIF2 and the accompanying reduction in the eIF2-GTP levels increase the time required for the scanning ribosomes to become competent to reinitiate translation. This delayed reinitiation allows for ribosomes to scan through the overlapping uORF2 and instead reinitiate at the ATF4-coding region. Physiologically, the increased expression of ATF4 contributes to the expression of genes involved in remediation of cellular stress damage (182). Along these lines, polymorphisms in the 5′ UTR that create or delete such uORFs may modulate the translation of the downstream coding sequences under conditions of stress. One such example involves a polymorphism in the 5′ UTR of excision repair 5 endonuclease (ERCC5) present in 35% of Caucasians (183). ERCC5 encodes for a protein directly involved in nucleotide excision repair, i.e., the DNA damage repair pathway that removes bulky DNA adducts induced by exposure to UV radiation and cisplatin. This common polymorphism results in the generation of an uORF in the 5′ UTR of ERCC5 mRNA which in turn is associated with upregulated ERCC5 expression following DNA damage. At the clinical level, this polymorphism has been associated with significantly lower progression free survival in pediatric patients with ependymoma treated with cisplatin-containing regimens (183).
Additionally, under conditions of stress when eIF2α phosphorylation diminishes significantly the availability of ternary complex, IRES-mediated translation (which does not require eIF2α) is upregulated (181). Under such conditions, Met-tRNAi may form an alternative ternary complex with factors like eIF5B, MCT-1, and ligatin (eIF2D) (181). Through this mechanism, cancer cells can circumvent the global downregulation of protein synthesis mediated by eIF2α phosphorylation and gain a survival advantage by the preferential translation of IRES-containing mRNAs. Transcript isoforms may contain or exclude an IRES as a result of alternative splicing and polymorphisms in the 5′ UTR may have functional implications in the cap-independent translation of the specific transcript. Indeed, two prevalent polymorphisms in the insulin like growth factor 1 receptor (IGF1R) 5′ UTR and more specifically the Loop3 poly(U)-tract of its IRES have functional implications in terms of translation initiation mediated through the IRES; minimizing the length of this poly(U)-tract has been associated with a consistent increase in the activity of the IRES (78). Collectively, eIF2α phosphorylation promotes the translation of select mRNAs that are inefficiently translated in the absence of stress (180). This shift in the translational landscape may explain why elevated levels of eIF2α phosphorylation correlate with cancer cell survival (181).
Regulation of Translation Elongation
Given its complexity and the requirement for the coordinate function of multiple factors, initiation has been traditionally thought as the rate-limiting step in translation. However, accumulating evidence suggests that translation can also be modulated at the level of elongation (184).
Multiple factors bind to the ribosomal A site during translation elongation and modulate translocation to the endoplasmic reticulum, polypeptide release, ribosome recycling, and mRNA decay (184). Ribosomes stall when the signal recognition particle (SRP) binds the N-terminus of the nascent polypeptide and docks into the ribosomal A site. Further tRNA entry is blocked and elongation is arrested until the ribosome/mRNA complex translocate to the endoplasmic reticulum (184). The SRP pathway is the best understood and thoroughly investigated pathway that regulates spatial organization and compartmentalization of translation (185) but other mechanisms also exist (186). Of note, recent studies have challenged the notion that only secretory proteins are translated in the endoplasmic reticulum; mRNAs encoding both cytosolic and topogenic signal-encoding proteins can be translated in the endoplasmic reticulum with similar translational efficiencies, which are consistently higher than the translational efficiencies in the cytosolic compartment (187).
Translation elongation may be modulated by factors acting on elements in the 3′ UTR. This is exemplified by hnRNP E1 which binds a 33-nucleotide TGF-B-activated translation (BAT) element (188, 189). This element is present on the 3′ UTR of transcripts that mediate epithelial–mesenchymal transition (EMT). Through this interaction, hnRNP E1 stalls translation elongation by inhibiting the release of eEF1A1 from the ribosomal A site. TGF-β-mediated hnRNP E1 phosphorylation, however, disrupts the BAT complex, thereby restoring translation elongation of the respective EMT-promoting transcripts (188, 189).
Translation elongation is also regulated by the phosphorylation status of eEF2 (190). During the stage of tumorigenesis, cells in whom oncogenic signaling pathways have been activated may rely on uninhibited translational elongation for malignant transformation (191). In fact, it is the enhanced translational elongation (rather than enhanced translation initiation) mediated by downregulation of the eEF2K and, consequently, activation of eEF2 that may underpin the increased protein synthesis during the stage of tumorigenesis (191). There have been contrasting reports about the role of eEF2K in cancer biology (95, 191, 192). By virtue of downregulating protein synthesis, high levels of eEF2K would be expected to impair proliferation. Indeed, the activity of eEF2K is inhibited by multiple oncogenic signals; however, high levels of eEF2K have been shown to increase the adaptability of tumor cells in nutrient deprivation accounting for the observation that elevated eEF2K transcript levels are associated with poor prognosis in medulloblastoma and glioblastoma multiforme (56) (Table 1; Table S1 in Supplementary Material).
rRNA Modifications Associated With Cancer: The Oncogenic Ribosome
Recent studies have challenged the view that ribosomes are constitutive components of the translational machinery with no regulatory function (79, 193). There is significant heterogeneity in ribosome composition that results from the differential expression and posttranslational modification of ribosomal proteins, rRNA diversity, and the activity of ribosome-associated factors (193). This ribosomal heterogeneity has a significant impact on the translational output of the transcriptional template (193). As outlined previously, DKC1 and FBL mediate the rRNA pseudouridylation (81) and methylation (18), respectively, which are important ribosomal modifications for IRES-mediated translation (18, 80, 81). Mutations or deletions of the DKC1 gene encoding DKC1 have a negative impact on the IRES-mediated translation of the tumor suppressor genes p53 and p27 predisposing to hematologic and solid malignancies (81). Altered rRNA methylation patterns mediated by FBL are associated with upregulated IRES-mediated translation of many oncogenes including IGF1R, MYC, FGF1, FGF2, and VEGFA (18). The expression of FBL is regulated by the tumor suppressor p53 and p53 inactivation, a common genomic alteration that occurs in multiple malignancies, leads to FBL upregulation. High expression of FBL mRNA has been associated with poor relapse-free and breast cancer-specific survival (18).
tRNA Abundance and Modifications Fostering Oncogenic Translation
The human genome contains 61 sense codons, many of which are synonymous on the basis that they encode the same amino acid (194). Synonymous codons are used with variable but non-random frequency across the genome constituting the basis of codon bias (195). Synonymous codons base pair with tRNA isoacceptors, i.e., tRNAs that are charged with the same amino acid but have a unique anticodon sequence. Normally, tRNA transcription via RNA polymerase III is regulated in response to nutrient availability and environmental cues, in coordination with rRNA transcription via RNA polymerase I (196). tRNAs have been typically considered as housekeeping products with little regulatory function; however, misregulation in tRNA abundance and modifications has been inherently linked to human disease including cancer (196).
In cancer, tRNAs are overexpressed to meet the demands of upregulated protein synthesis. Indeed, oncogenes such as c-myc and tumor suppressor genes such as p53 and RB, regulate positively and negatively, respectively, the transcription of RNA polymerase III (197). The transcription of RNA polymerase III (and consequently tRNA synthesis) is also modulated by oncogenic signaling pathways; ERK phosphorylates the transcription factor TFIIIB, which in turn upregulates the transcription of RNA polymerase III (198), while mTORC1 phosphorylates and inactivates MAF1, a repressor of RNA polymerase III activity (172).
Multiple lines of evidence suggest that the implications of upregulated tRNA synthesis in cancer go beyond the simple need to meet the demands of increased protein synthesis. Experimentally, overexpression of Met-tRNAi in human breast epithelial cells reprogrammed the tRNA pool and led to increased metabolic activity and proliferation rates (196). Studies show that changes in the tRNA pool and composition are neither uniform nor random; tRNA overexpression is selective and coordinates with the status of the cell to favor a specific translational program (199, 200). Indeed, malignant proliferating cells and non-malignant differentiating cells have distinct tRNA pools whose anticodon composition suits the codon usage signature of proliferation/cancer-related and differentiation-related/housekeeping genes, respectively (199, 200). In this context, assessment of tRNA pools by tRNA microarrays or other methodologies holds promise as a biomarker to determine the natural history of a tumor or malignant potential of a premalignant lesion.
tRNAs also undergo extensive posttranscriptional modifications; these dynamic modifications have been traditionally linked with an adaptive stress response, whereby mRNA translation is rapidly suppressed or altered (201, 202). However, several recent studies link these modifications with cancer. This is exemplified by the role of the TRM6/61 methyltransferase complex, which mediates the methylation of adenosine at position 58 of Met-tRNAi (57). This important modification stabilizes Met-tRNAi, which in turn allows for the selective translation of oncogenic transcripts. Increasing levels of TRM6/61 correlate with the transition from grade II or III gliomas to glioblastomas, i.e., tumors of the central nervous system with progressively more aggressive natural history (57). The elongator acetyltransferase complex subunit 3 and CTU1/2 enzymes mediate the posttranscriptional modification of the wobble uridine 34, a highly conserved modification that contributes to translational fidelity (19). Increasing levels of those enzymes correlated with the transition from normal breast tissue to non-invasive and invasive breast cancer. This posttranscriptional modification is important for the translation of an ITAF (DEK proto-oncogene), which in turn upregulates the IRES-mediated translation of the transcription factor lymphoid enhancer binding factor 1 (LEF1) (19); of note, LEF1 is an important effector of the WNT- and TGFβ-signaling pathways, which lead to invasion and metastases. On the other hand, the isopentenyltransferase TRIT1, which catalyzes the addition of N6-isopentenyladenosine on residue 37 of tRNAs, is a tumor suppressor gene in lung cancer (24).
Aminoacyl-tRNA synthetases catalyze the ligation of amino acids to their cognate tRNAs (203). Some of the synthetases are tightly bound together in a large multisynthetase complex, with three tRNA synthetase associated proteins at the core designated as aminoacyl-tRNA synthetase-interacting multifunctional protein (AIMP) 1, 2, and 3 (203). Eukaryotic synthetases contain unique extensions and domains, which, alongside with variable subcellular localization (nuclear, cytoplasmic, or secreted) either within complexes or free, endows them with an increasingly recognized functional diversity extending beyond translation (203, 204). Although the importance of the canonical functions of the tRNA synthetases in cancer has been recognized early (205, 206), it is the additional non-canonical activities that are more indicative of a role in tumorigenesis (207).
Concluding Remarks and Future Perspectives
Collectively, over the recent years, the complexity of posttranscriptional regulation of gene expression is becoming increasingly recognized. New technologies and advances in next-generation sequencing have allowed us to dissect the mechanistic underpinnings of translational regulation at an unprecedented scale and resolution (93). Translation becomes distinctively dysregulated in cancer and this dysregulation is critical for oncogenes and oncogenic signaling pathways to carry out their transformative potential while at the same time, endows cancer cells with distinctive adaptive capabilities to a diverse nature of stresses.
In this context, multiple factors involved in the posttranscriptional regulation of gene expression arise as biomarkers with potential diagnostic, prognostic, or predictive utility. Assessment of these factors can help us in the diagnosis of equivocal cases, determine the malignant potential of premalignant lesions, predict response to a specific therapy, determine the risk of recurrence or cancer-related death, and altogether help us in clinical decision making and the refinement of our treatment approaches. Rapidly emerging new data are likely to provide even greater insight and rationale for the utilization of these translational regulatory factors in patient stratification and treatment selection. Moreover, as new drugs targeting the translational machinery enter clinical investigations, the role and importance of those biomarkers are expected to expand.
Author Contributions
WG conceived the manuscript. CV collected the data and drafted the manuscript. WG and SB critically reviewed the manuscript. All authors approved the final version to be published.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The handling editor declared a shared affiliation, though no other collaboration, with the authors and the handling editor states that the process met the standards of a fair and objective review.
Acknowledgments
We thank Dr. Sunnie R Thompson for critical review of the manuscript and insightful comments.
Footnotes
Funding. CV is currently funded by Career Catalyst Award in Basic and Translational Research by Susan G Komen (CCR15331062).
Supplementary Material
The Supplementary Material for this article can be found online at http://journal.frontiersin.org/article/10.3389/fonc.2017.00158/full#supplementary-material.
Abbreviations
4E-BP, eIF4E-binding protein; A, adenosine; A-site, acceptor site; ABCE1, ATP-binding cassette subfamily E member 1; ADAR, adenosine deaminase, RNA-specific; ADARB1/2, adenosine deaminase, RNA-specific B1/2; AIMP, aminoacyl-tRNA synthetase-interacting multifunctional protein; AML, acute myelogenous leukemia; APAF1, apoptotic protease activating factor; ARE, AU-rich element; ATF4, activating transcription factor 4; BAT, TGF-B-activated translation element; BRAF, B-Raf proto-oncogene, serine/threonine kinase; Bcl-2, Bcl-2 apoptosis regulator; BTG1, BTG anti-proliferation factor 1; C/EBPα, CCAAT/enhancer binding protein alpha; CDK6, cyclin dependent kinase 6; CDK12, cyclin dependent kinase 12; CPE, cytoplasmic polyadenylation element; CPEB1–4, cytoplasmic polyadenylation element-binding protein 1–4; c-Jun, Jun proto-oncogene; c-myc, v-myc avian myelocytomatosis viral oncogene homolog; CTU1/2, cytosolic thiouridylase subunit 1/2; DEAD, Asp-Glu-Ala-Asp (tetrapeptide shared by the DEAD-box family of proteins); DEK, DEK proto-oncogene; DKC1, dyskerin; DNA, deoxyribonucleic acid; eEF1/2, eukaryotic translation elongation factor 1/2; eEF2K, eEF2 kinase; eIF (1, 1A, 2, 2α, 2B, 2D, 3, 3d, 4A, 4B, 4F, 5, 5B), eukaryotic initiation factor (1, 1A, 2, 2α, 2B, 2D, 3, 3d, 4A, 4B, 4F, 5, 5B); ELP3, elongator acetyltransferase complex subunit 3; EMT, epithelial–mesenchymal transition; ER, estrogen receptor; ERCC5, excision repair 5 endonuclease; eRF1/3, eukaryotic translation termination factor 1/3; ERK1, mitogen-activated protein kinase 3; ERK2, mitogen-activated protein kinase 1; FBL, fibrillarin; FGF1/2, fibroblast growth factor 1/2; FTO, alpha-ketoglutarate-dependent dioxygenase; GCN2, general control non-derepressible 2 kinase; GDP, guanosine-5’-diphosphate; GLD-1, female germline-specific tumor suppressor gld-1; GTP, guanosine-5’-triphosphate; HIF1α/2α, hypoxia-inducible factor 1α/2α; hm5C, 5-hydroxylmethylcytosine; hnRNP A1/C, heterogeneous nuclear ribonucleoprotein A1/C; hnRNP E1, poly(rC)-binding protein 1; HRI, heme-regulated eIF2α kinase; HSP90AB1, heat shock protein 90 alpha family class B member 1; HuR, ELAV-like RNA-binding protein 1; I, inosine; IGF1R, insulin-like growth factor 1 receptor; IGF2BP1/3, insulin-like growth factor 2 mRNA-binding protein 1/3; IRES, internal ribosome entry site; ITAF, IRES trans-acting factor; LEF1, lymphoid enhancer binding factor 1; m7Gppp, 7-methyl guanosine; KRAS, KRAS proto-oncogene GTPase; m1A, N1-methyladenosine; m5C, 5-methylcytosine; m6A, N6-methyladenosine; m6Am, N6,2’-O-dimethyladenosine; MAF1, MAF1 homolog, negative regulator of RNA polymerase III; MCT-1/DENR, multiple copies in T-cell lymphoma-1 and density regulated protein; MEK, mitogen-activated protein kinase kinase 7; Met, methionine; Met-tRNA, methionyl tRNA; mRNA, messenger RNA; MDR1, ATP-binding cassette subfamily B member 1; MNK1/2, MAP kinase interacting serine/threonine kinase 1/2; mTOR, mechanistic or mammalian target of rapamycin; mTORC1/2, mTORcomplex 1/2; NF-κB, nuclear factor kappa B; NPM1, nucleophosmin; ORF, open reading frame; p38 MAP, mitogen-activated protein kinase 14; p53, tumor protein p53; PAR-CLIP, photoactivatable ribonucleoside-enhanced crosslinking and immunoprecipitation; P-site, peptidyl site; PABP, poly(A)-binding protein; PDCD4, programmed cell death 4; PERK, PKR-like endoplasmic reticulum kinase; PKR, protein kinase RNA-activated; RB, RB transcriptional corepressor 1; RNA, ribonucleic acid; RPL38, ribosomal protein L38; RPS25, ribosomal protein S25; rRNA, ribosome RNA; S6K, ribosomal protein S6 kinase; SRP, signal recognition particle; TFIIIB, subunit of RNA polymerase III transcription initiation factor IIIB; TGFβ, transforming growth factor beta; TIE, translation inhibitory element; TIF-1A, tripartite motif containing 24; TRIT1, tRNA isopentenyltransferase 1; TRM6/61, tRNA methyltransferase 61A; tRNA, transfer RNA; uORF, upstream ORF; UTR, untranslated region; UV, ultraviolet; VEGF, vascular endothelial growth factor; XIAP, X-linked inhibitor of apoptosis; YB-1, Y-box binding protein 1; ψ, pseudouridine.
References
- 1.Schwanhausser B, Busse D, Li N, Dittmar G, Schuchhardt J, Wolf J, et al. Global quantification of mammalian gene expression control. Nature (2011) 473(7347):337–42. 10.1038/nature10098 [DOI] [PubMed] [Google Scholar]
- 2.Sonenberg N, Hinnebusch AG. Regulation of translation initiation in eukaryotes: mechanisms and biological targets. Cell (2009) 136(4):731–45. 10.1016/j.cell.2009.01.042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rajasekhar VK, Viale A, Socci ND, Wiedmann M, Hu X, Holland EC. Oncogenic Ras and Akt signaling contribute to glioblastoma formation by differential recruitment of existing mRNAs to polysomes. Mol Cell (2003) 12(4):889–901. 10.1016/S1097-2765(03)00395-2 [DOI] [PubMed] [Google Scholar]
- 4.Boussemart L, Malka-Mahieu H, Girault I, Allard D, Hemmingsson O, Tomasic G, et al. eIF4F is a nexus of resistance to anti-BRAF and anti-MEK cancer therapies. Nature (2014) 513(7516):105–9. 10.1038/nature13572 [DOI] [PubMed] [Google Scholar]
- 5.Cammas A, Lacroix-Triki M, Pierredon S, Le Bras M, Iacovoni JS, Teulade-Fichou MP, et al. hnRNP A1-mediated translational regulation of the G quadruplex-containing RON receptor tyrosine kinase mRNA linked to tumor progression. Oncotarget (2016) 7(13):16793–805. 10.18632/oncotarget.7589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Habibi G, Leung S, Law JH, Gelmon K, Masoudi H, Turbin D, et al. Redefining prognostic factors for breast cancer: YB-1 is a stronger predictor of relapse and disease-specific survival than estrogen receptor or HER-2 across all tumor subtypes. Breast Cancer Res (2008) 10(5):R86. 10.1186/bcr2156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Heinonen M, Bono P, Narko K, Chang SH, Lundin J, Joensuu H, et al. Cytoplasmic HuR expression is a prognostic factor in invasive ductal breast carcinoma. Cancer Res (2005) 65(6):2157–61. 10.1158/0008-5472.CAN-04-3765 [DOI] [PubMed] [Google Scholar]
- 8.Heinonen M, Fagerholm R, Aaltonen K, Kilpivaara O, Aittomaki K, Blomqvist C, et al. Prognostic role of HuR in hereditary breast cancer. Clin Cancer Res (2007) 13(23):6959–63. 10.1158/1078-0432.CCR-07-1432 [DOI] [PubMed] [Google Scholar]
- 9.Walter O, Prasad M, Lu S, Quinlan RM, Edmiston KL, Khan A. IMP3 is a novel biomarker for triple negative invasive mammary carcinoma associated with a more aggressive phenotype. Hum Pathol (2009) 40(11):1528–33. 10.1016/j.humpath.2009.05.005 [DOI] [PubMed] [Google Scholar]
- 10.Zhou S, Wang GP, Liu C, Zhou M. Eukaryotic initiation factor 4E (eIF4E) and angiogenesis: prognostic markers for breast cancer. BMC Cancer (2006) 6:231. 10.1186/1471-2407-6-231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Flowers A, Chu QD, Panu L, Meschonat C, Caldito G, Lowery-Nordberg M, et al. Eukaryotic initiation factor 4E overexpression in triple-negative breast cancer predicts a worse outcome. Surgery (2009) 146(2):220–6. 10.1016/j.surg.2009.05.010 [DOI] [PubMed] [Google Scholar]
- 12.Modelska A, Turro E, Russell R, Beaton J, Sbarrato T, Spriggs K, et al. The malignant phenotype in breast cancer is driven by eIF4A1-mediated changes in the translational landscape. Cell Death Dis (2015) 6:e1603. 10.1038/cddis.2014.542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McClusky DR, Chu Q, Yu H, Debenedetti A, Johnson LW, Meschonat C, et al. A prospective trial on initiation factor 4E (eIF4E) overexpression and cancer recurrence in node-positive breast cancer. Ann Surg (2005) 242(4):584–90; discussion 90–2. 10.1097/01.sla.0000184224.55949.90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yin X, Kim RH, Sun G, Miller JK, Li BD. Overexpression of eukaryotic initiation factor 4E is correlated with increased risk for systemic dissemination in node-positive breast cancer patients. J Am Coll Surg (2014) 218(4):663–71. 10.1016/j.jamcollsurg.2013.12.020 [DOI] [PubMed] [Google Scholar]
- 15.Li BD, Gruner JS, Abreo F, Johnson LW, Yu H, Nawas S, et al. Prospective study of eukaryotic initiation factor 4E protein elevation and breast cancer outcome. Ann Surg (2002) 235(5):732–8; discussion 8–9. 10.1097/00000658-200205000-00016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rojo F, Najera L, Lirola J, Jimenez J, Guzman M, Sabadell MD, et al. 4E-binding protein 1, a cell signaling hallmark in breast cancer that correlates with pathologic grade and prognosis. Clin Cancer Res (2007) 13(1):81–9. 10.1158/1078-0432.CCR-06-1560 [DOI] [PubMed] [Google Scholar]
- 17.Silvera D, Arju R, Darvishian F, Levine PH, Zolfaghari L, Goldberg J, et al. Essential role for eIF4GI overexpression in the pathogenesis of inflammatory breast cancer. Nat Cell Biol (2009) 11(7):903–8. 10.1038/ncb1900 [DOI] [PubMed] [Google Scholar]
- 18.Marcel V, Ghayad SE, Belin S, Therizols G, Morel AP, Solano-Gonzalez E, et al. p53 acts as a safeguard of translational control by regulating fibrillarin and rRNA methylation in cancer. Cancer Cell (2013) 24(3):318–30. 10.1016/j.ccr.2013.08.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Delaunay S, Rapino F, Tharun L, Zhou Z, Heukamp L, Termathe M, et al. Elp3 links tRNA modification to IRES-dependent translation of LEF1 to sustain metastasis in breast cancer. J Exp Med (2016) 213(11):2503–23. 10.1084/jem.20160397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhao S, Guo W, Li J, Yu W, Guo T, Deng W, et al. High expression of Y-box-binding protein 1 correlates with poor prognosis and early recurrence in patients with small invasive lung adenocarcinoma. Onco Targets Ther (2016) 9:2683–92. 10.2147/OTT.S99939 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang J, Wang B, Bi J, Zhang C. Cytoplasmic HuR expression correlates with angiogenesis, lymphangiogenesis, and poor outcome in lung cancer. Med Oncol (2011) 28(Suppl 1):S577–85. 10.1007/s12032-010-9734-6 [DOI] [PubMed] [Google Scholar]
- 22.Kim GY, Lim SJ, Kim YW. Expression of HuR, COX-2, and survivin in lung cancers; cytoplasmic HuR stabilizes cyclooxygenase-2 in squamous cell carcinomas. Mod Pathol (2011) 24(10):1336–47. 10.1038/modpathol.2011.90 [DOI] [PubMed] [Google Scholar]
- 23.Chen Y, Knosel T, Kristiansen G, Pietas A, Garber ME, Matsuhashi S, et al. Loss of PDCD4 expression in human lung cancer correlates with tumour progression and prognosis. J Pathol (2003) 200(5):640–6. 10.1002/path.1378 [DOI] [PubMed] [Google Scholar]
- 24.Spinola M, Galvan A, Pignatiello C, Conti B, Pastorino U, Nicander B, et al. Identification and functional characterization of the candidate tumor suppressor gene TRIT1 in human lung cancer. Oncogene (2005) 24(35):5502–9. 10.1038/sj.onc.1208687 [DOI] [PubMed] [Google Scholar]
- 25.Zhou ZJ, Dai Z, Zhou SL, Fu XT, Zhao YM, Shi YH, et al. Overexpression of HnRNP A1 promotes tumor invasion through regulating CD44v6 and indicates poor prognosis for hepatocellular carcinoma. Int J Cancer (2013) 132(5):1080–9. 10.1002/ijc.27742 [DOI] [PubMed] [Google Scholar]
- 26.Shiraiwa S, Kinugasa T, Kawahara A, Mizobe T, Ohchi T, Yuge K, et al. Nuclear Y-box-binding protein-1 expression predicts poor clinical outcome in stage III colorectal cancer. Anticancer Res (2016) 36(7):3781–8. [PubMed] [Google Scholar]
- 27.Shinkai K, Nakano K, Cui L, Mizuuchi Y, Onishi H, Oda Y, et al. Nuclear expression of Y-box binding protein-1 is associated with poor prognosis in patients with pancreatic cancer and its knockdown inhibits tumor growth and metastasis in mice tumor models. Int J Cancer (2016) 139(2):433–45. 10.1002/ijc.30075 [DOI] [PubMed] [Google Scholar]
- 28.Zhang Y, Zhao PW, Feng G, Xie G, Wang AQ, Yang YH, et al. The expression level and prognostic value of Y-box binding protein-1 in rectal cancer. PLoS One (2015) 10(3):e0119385. 10.1371/journal.pone.0119385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tatarian T, Jiang W, Leiby BE, Grigoli A, Jimbo M, Dabbish N, et al. Cytoplasmic HuR status predicts disease-free survival in resected pancreatic cancer: a post-hoc analysis from the international phase III ESPAC-3 clinical trial. Ann Surg (2016). 10.1097/SLA.0000000000002088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mrena J, Wiksten JP, Thiel A, Kokkola A, Pohjola L, Lundin J, et al. Cyclooxygenase-2 is an independent prognostic factor in gastric cancer and its expression is regulated by the messenger RNA stability factor HuR. Clin Cancer Res (2005) 11(20):7362–8. 10.1158/1078-0432.CCR-05-0764 [DOI] [PubMed] [Google Scholar]
- 31.Denkert C, Koch I, von Keyserlingk N, Noske A, Niesporek S, Dietel M, et al. Expression of the ELAV-like protein HuR in human colon cancer: association with tumor stage and cyclooxygenase-2. Mod Pathol (2006) 19(9):1261–9. 10.1038/modpathol.3800645 [DOI] [PubMed] [Google Scholar]
- 32.Okada K, Fujiwara Y, Nakamura Y, Takiguchi S, Nakajima K, Miyata H, et al. Oncofetal protein, IMP-3, a potential marker for prediction of postoperative peritoneal dissemination in gastric adenocarcinoma. J Surg Oncol (2012) 105(8):780–5. 10.1002/jso.22108 [DOI] [PubMed] [Google Scholar]
- 33.Chen YL, Jeng YM, Hsu HC, Lai HS, Lee PH, Lai PL, et al. Expression of insulin-like growth factor II mRNA-binding protein 3 predicts early recurrence and poor prognosis in intrahepatic cholangiocarcinoma. Int J Surg (2013) 11(1):85–91. 10.1016/j.ijsu.2012.11.021 [DOI] [PubMed] [Google Scholar]
- 34.Li D, Yan D, Tang H, Zhou C, Fan J, Li S, et al. IMP3 is a novel prognostic marker that correlates with colon cancer progression and pathogenesis. Ann Surg Oncol (2009) 16(12):3499–506. 10.1245/s10434-009-0648-5 [DOI] [PubMed] [Google Scholar]
- 35.Lin L, Zhang J, Wang Y, Ju W, Ma Y, Li L, et al. Insulin-like growth factor-II mRNA-binding protein 3 predicts a poor prognosis for colorectal adenocarcinoma. Oncol Lett (2013) 6(3):740–4. 10.3892/ol.2013.1458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Diab-Assaf M, Abou-Khouzam R, Saadallah-Zeidan N, Habib K, Bitar N, Karam W, et al. Expression of eukaryotic initiation factor 4E and 4E binding protein 1 in colorectal carcinogenesis. Int J Clin Exp Pathol (2015) 8(1):404–13. [PMC free article] [PubMed] [Google Scholar]
- 37.Chen CN, Hsieh FJ, Cheng YM, Lee PH, Chang KJ. Expression of eukaryotic initiation factor 4E in gastric adenocarcinoma and its association with clinical outcome. J Surg Oncol (2004) 86(1):22–7. 10.1002/jso.20037 [DOI] [PubMed] [Google Scholar]
- 38.Chao MW, Wang LT, Lai CY, Yang XM, Cheng YW, Lee KH, et al. eIF4E binding protein 1 expression is associated with clinical survival outcomes in colorectal cancer. Oncotarget (2015) 6(27):24092–104. 10.18632/oncotarget.4483 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fassan M, Cagol M, Pennelli G, Rizzetto C, Giacomelli L, Battaglia G, et al. Programmed cell death 4 protein in esophageal cancer. Oncol Rep (2010) 24(1):135–9. 10.3892/or_00000838 [DOI] [PubMed] [Google Scholar]
- 40.Mudduluru G, Medved F, Grobholz R, Jost C, Gruber A, Leupold JH, et al. Loss of programmed cell death 4 expression marks adenoma-carcinoma transition, correlates inversely with phosphorylated protein kinase B, and is an independent prognostic factor in resected colorectal cancer. Cancer (2007) 110(8):1697–707. 10.1002/cncr.22983 [DOI] [PubMed] [Google Scholar]
- 41.Fassan M, Pizzi M, Battaglia G, Giacomelli L, Parente P, Bocus P, et al. Programmed cell death 4 (PDCD4) expression during multistep Barrett’s carcinogenesis. J Clin Pathol (2010) 63(8):692–6. 10.1136/jcp.2010.078253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Huang H, Han Y, Zhang C, Wu J, Feng J, Qu L, et al. HNRNPC as a candidate biomarker for chemoresistance in gastric cancer. Tumour Biol (2016) 37(3):3527–34. 10.1007/s13277-015-4144-1 [DOI] [PubMed] [Google Scholar]
- 43.Shiota M, Fujimoto N, Imada K, Yokomizo A, Itsumi M, Takeuchi A, et al. Potential role for YB-1 in castration-resistant prostate cancer and resistance to enzalutamide through the androgen receptor V7. J Natl Cancer Inst (2016) 108(7):djw005. 10.1093/jnci/djw005 [DOI] [PubMed] [Google Scholar]
- 44.Furic L, Rong L, Larsson O, Koumakpayi IH, Yoshida K, Brueschke A, et al. eIF4E phosphorylation promotes tumorigenesis and is associated with prostate cancer progression. Proc Natl Acad Sci U S A (2010) 107(32):14134–9. 10.1073/pnas.1005320107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nishio S, Ushijima K, Yamaguchi T, Sasajima Y, Tsuda H, Kasamatsu T, et al. Nuclear Y-box-binding protein-1 is a poor prognostic marker and related to epidermal growth factor receptor in uterine cervical cancer. Gynecol Oncol (2014) 132(3):703–8. 10.1016/j.ygyno.2014.01.045 [DOI] [PubMed] [Google Scholar]
- 46.Denkert C, Weichert W, Pest S, Koch I, Licht D, Kobel M, et al. Overexpression of the embryonic-lethal abnormal vision-like protein HuR in ovarian carcinoma is a prognostic factor and is associated with increased cyclooxygenase 2 expression. Cancer Res (2004) 64(1):189–95. 10.1158/0008-5472.CAN-03-1987 [DOI] [PubMed] [Google Scholar]
- 47.Lim SJ, Kim HJ, Kim JY, Park K, Lee CM. Expression of HuR is associated with increased cyclooxygenase-2 expression in uterine cervical carcinoma. Int J Gynecol Pathol (2007) 26(3):229–34. 10.1097/01.pgp.0000236946.82334.07 [DOI] [PubMed] [Google Scholar]
- 48.Kobel M, Weidensdorfer D, Reinke C, Lederer M, Schmitt WD, Zeng K, et al. Expression of the RNA-binding protein IMP1 correlates with poor prognosis in ovarian carcinoma. Oncogene (2007) 26(54):7584–9. 10.1038/sj.onc.1210563 [DOI] [PubMed] [Google Scholar]
- 49.Kobel M, Xu H, Bourne PA, Spaulding BO, Shih Ie M, Mao TL, et al. IGF2BP3 (IMP3) expression is a marker of unfavorable prognosis in ovarian carcinoma of clear cell subtype. Mod Pathol (2009) 22(3):469–75. 10.1038/modpathol.2008.206 [DOI] [PubMed] [Google Scholar]
- 50.Fadare O, Liang SX, Crispens MA, Jones HW, III, Khabele D, Gwin K, et al. Expression of the oncofetal protein IGF2BP3 in endometrial clear cell carcinoma: assessment of frequency and significance. Hum Pathol (2013) 44(8):1508–15. 10.1016/j.humpath.2012.12.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Castellvi J, Garcia A, Ruiz-Marcellan C, Hernandez-Losa J, Peg V, Salcedo M, et al. Cell signaling in endometrial carcinoma: phosphorylated 4E-binding protein-1 expression in endometrial cancer correlates with aggressive tumors and prognosis. Hum Pathol (2009) 40(10):1418–26. 10.1016/j.humpath.2008.12.019 [DOI] [PubMed] [Google Scholar]
- 52.Wang X, Wei Z, Gao F, Zhang X, Zhou C, Zhu F, et al. Expression and prognostic significance of PDCD4 in human epithelial ovarian carcinoma. Anticancer Res (2008) 28(5B):2991–6. [PubMed] [Google Scholar]
- 53.Zheng J, Dong W, Zhang J, Li G, Gong H. YB-1, a new biomarker of glioma progression, is associated with the prognosis of glioma patients. Acta Biochim Biophys Sin (Shanghai) (2016) 48(4):318–25. 10.1093/abbs/gmw012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bell JL, Turlapati R, Liu T, Schulte JH, Huttelmaier S. IGF2BP1 harbors prognostic significance by gene gain and diverse expression in neuroblastoma. J Clin Oncol (2015) 33(11):1285–93. 10.1200/JCO.2014.55.9880 [DOI] [PubMed] [Google Scholar]
- 55.Gao F, Wang X, Zhu F, Wang Q, Zhang X, Guo C, et al. PDCD4 gene silencing in gliomas is associated with 5’CpG island methylation and unfavourable prognosis. J Cell Mol Med (2009) 13(10):4257–67. 10.1111/j.1582-4934.2008.00497.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Leprivier G, Remke M, Rotblat B, Dubuc A, Mateo AR, Kool M, et al. The eEF2 kinase confers resistance to nutrient deprivation by blocking translation elongation. Cell (2013) 153(5):1064–79. 10.1016/j.cell.2013.04.055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Macari F, El-Houfi Y, Boldina G, Xu H, Khoury-Hanna S, Ollier J, et al. TRM6/61 connects PKCalpha with translational control through tRNAi(Met) stabilization: impact on tumorigenesis. Oncogene (2016) 35(14):1785–96. 10.1038/onc.2015.244 [DOI] [PubMed] [Google Scholar]
- 58.Jackson RJ, Hellen CU, Pestova TV. The mechanism of eukaryotic translation initiation and principles of its regulation. Nat Rev Mol Cell Biol (2010) 11(2):113–27. 10.1038/nrm2838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Choe J, Ryu I, Park OH, Park J, Cho H, Yoo JS, et al. eIF4AIII enhances translation of nuclear cap-binding complex-bound mRNAs by promoting disruption of secondary structures in 5’UTR. Proc Natl Acad Sci U S A (2014) 111(43):E4577–86. 10.1073/pnas.1409695111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Pelletier J, Graff J, Ruggero D, Sonenberg N. Targeting the eIF4F translation initiation complex: a critical nexus for cancer development. Cancer Res (2015) 75(2):250–63. 10.1158/0008-5472.CAN-14-2789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Galicia-Vazquez G, Cencic R, Robert F, Agenor AQ, Pelletier J. A cellular response linking eIF4AI activity to eIF4AII transcription. RNA (2012) 18(7):1373–84. 10.1261/rna.033209.112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Parsyan A, Svitkin Y, Shahbazian D, Gkogkas C, Lasko P, Merrick WC, et al. mRNA helicases: the tacticians of translational control. Nat Rev Mol Cell Biol (2011) 12(4):235–45. 10.1038/nrm3083 [DOI] [PubMed] [Google Scholar]
- 63.Rogers GW, Jr, Richter NJ, Merrick WC. Biochemical and kinetic characterization of the RNA helicase activity of eukaryotic initiation factor 4A. J Biol Chem (1999) 274(18):12236–44. 10.1074/jbc.274.18.12236 [DOI] [PubMed] [Google Scholar]
- 64.Rozovsky N, Butterworth AC, Moore MJ. Interactions between eIF4AI and its accessory factors eIF4B and eIF4H. RNA (2008) 14(10):2136–48. 10.1261/rna.1049608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Feoktistova K, Tuvshintogs E, Do A, Fraser CS. Human eIF4E promotes mRNA restructuring by stimulating eIF4A helicase activity. Proc Natl Acad Sci U S A (2013) 110(33):13339–44. 10.1073/pnas.1303781110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Bhat M, Robichaud N, Hulea L, Sonenberg N, Pelletier J, Topisirovic I. Targeting the translation machinery in cancer. Nat Rev Drug Discov (2015) 14(4):261–78. 10.1038/nrd4505 [DOI] [PubMed] [Google Scholar]
- 67.von der Haar T, Gross JD, Wagner G, McCarthy JE. The mRNA cap-binding protein eIF4E in post-transcriptional gene expression. Nat Struct Mol Biol (2004) 11(6):503–11. 10.1038/nsmb779 [DOI] [PubMed] [Google Scholar]
- 68.Lee AS, Kranzusch PJ, Doudna JA, Cate JH. eIF3d is an mRNA cap-binding protein that is required for specialized translation initiation. Nature (2016) 536(7614):96–9. 10.1038/nature18954 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Uniacke J, Holterman CE, Lachance G, Franovic A, Jacob MD, Fabian MR, et al. An oxygen-regulated switch in the protein synthesis machinery. Nature (2012) 486(7401):126–9. 10.1038/nature11055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Uniacke J, Perera JK, Lachance G, Francisco CB, Lee S. Cancer cells exploit eIF4E2-directed synthesis of hypoxia response proteins to drive tumor progression. Cancer Res (2014) 74(5):1379–89. 10.1158/0008-5472.CAN-13-2278 [DOI] [PubMed] [Google Scholar]
- 71.Timpano S, Uniacke J. Human cells cultured under physiological oxygen utilize two cap-binding proteins to recruit distinct mRNAs for translation. J Biol Chem (2016) 291(20):10772–82. 10.1074/jbc.M116.717363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Lee AS, Kranzusch PJ, Cate JH. eIF3 targets cell-proliferation messenger RNAs for translational activation or repression. Nature (2015) 522(7554):111–4. 10.1038/nature14267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hinnebusch AG. The scanning mechanism of eukaryotic translation initiation. Annu Rev Biochem (2014) 83:779–812. 10.1146/annurev-biochem-060713-035802 [DOI] [PubMed] [Google Scholar]
- 74.King HA, Cobbold LC, Willis AE. The role of IRES trans-acting factors in regulating translation initiation. Biochem Soc Trans (2010) 38(6):1581–6. 10.1042/BST0381581 [DOI] [PubMed] [Google Scholar]
- 75.Walters B, Thompson SR. Cap-independent translational control of carcinogenesis. Front Oncol (2016) 6:128. 10.3389/fonc.2016.00128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Komar AA, Hatzoglou M. Cellular IRES-mediated translation: the war of ITAFs in pathophysiological states. Cell Cycle (2011) 10(2):229–40. 10.4161/cc.10.2.14472 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Chappell SA, Edelman GM, Mauro VP. Biochemical and functional analysis of a 9-nt RNA sequence that affects translation efficiency in eukaryotic cells. Proc Natl Acad Sci U S A (2004) 101(26):9590–4. 10.1073/pnas.0308759101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Meng Z, Jackson NL, Shcherbakov OD, Choi H, Blume SW. The human IGF1R IRES likely operates through a Shine-Dalgarno-like interaction with the G961 loop (E-site) of the 18S rRNA and is kinetically modulated by a naturally polymorphic polyU loop. J Cell Biochem (2010) 110(2):531–44. 10.1002/jcb.22569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Xue S, Tian S, Fujii K, Kladwang W, Das R, Barna M. RNA regulons in Hox 5’UTRs confer ribosome specificity to gene regulation. Nature (2015) 517(7532):33–8. 10.1038/nature14010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Jack K, Bellodi C, Landry DM, Niederer RO, Meskauskas A, Musalgaonkar S, et al. rRNA pseudouridylation defects affect ribosomal ligand binding and translational fidelity from yeast to human cells. Mol Cell (2011) 44(4):660–6. 10.1016/j.molcel.2011.09.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Ruggero D, Grisendi S, Piazza F, Rego E, Mari F, Rao PH, et al. Dyskeratosis congenita and cancer in mice deficient in ribosomal RNA modification. Science (2003) 299(5604):259–62. 10.1126/science.1079447 [DOI] [PubMed] [Google Scholar]
- 82.Hertz MI, Landry DM, Willis AE, Luo G, Thompson SR. Ribosomal protein S25 dependency reveals a common mechanism for diverse internal ribosome entry sites and ribosome shunting. Mol Cell Biol (2013) 33(5):1016–26. 10.1128/MCB.00879-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Spriggs KA, Bushell M, Willis AE. Translational regulation of gene expression during conditions of cell stress. Mol Cell (2010) 40(2):228–37. 10.1016/j.molcel.2010.09.028 [DOI] [PubMed] [Google Scholar]
- 84.Huez I, Creancier L, Audigier S, Gensac MC, Prats AC, Prats H. Two independent internal ribosome entry sites are involved in translation initiation of vascular endothelial growth factor mRNA. Mol Cell Biol (1998) 18(11):6178–90. 10.1128/MCB.18.11.6178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Sherrill KW, Byrd MP, Van Eden ME, Lloyd RE. BCL-2 translation is mediated via internal ribosome entry during cell stress. J Biol Chem (2004) 279(28):29066–74. 10.1074/jbc.M402727200 [DOI] [PubMed] [Google Scholar]
- 86.Martineau Y, Le Bec C, Monbrun L, Allo V, Chiu IM, Danos O, et al. Internal ribosome entry site structural motifs conserved among mammalian fibroblast growth factor 1 alternatively spliced mRNAs. Mol Cell Biol (2004) 24(17):7622–35. 10.1128/MCB.24.17.7622-7635.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Blau L, Knirsh R, Ben-Dror I, Oren S, Kuphal S, Hau P, et al. Aberrant expression of c-Jun in glioblastoma by internal ribosome entry site (IRES)-mediated translational activation. Proc Natl Acad Sci U S A (2012) 109(42):E2875–84. 10.1073/pnas.1203659109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Dobson T, Chen J, Krushel LA. Dysregulating IRES-dependent translation contributes to over-expression of the aurora A kinase onco-protein. Mol Cancer Res (2013) 11(8):887–900. 10.1158/1541-7786.MCR-12-0707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Nanbru C, Lafon I, Audigier S, Gensac MC, Vagner S, Huez G, et al. Alternative translation of the proto-oncogene c-myc by an internal ribosome entry site. J Biol Chem (1997) 272(51):32061–6. 10.1074/jbc.272.51.32061 [DOI] [PubMed] [Google Scholar]
- 90.Stoneley M, Paulin FE, Le Quesne JP, Chappell SA, Willis AE. C-Myc 5’ untranslated region contains an internal ribosome entry segment. Oncogene (1998) 16(3):423–8. 10.1038/sj.onc.1201763 [DOI] [PubMed] [Google Scholar]
- 91.Meng Z, King PH, Nabors LB, Jackson NL, Chen CY, Emanuel PD, et al. The ELAV RNA-stability factor HuR binds the 5’-untranslated region of the human IGF-IR transcript and differentially represses cap-dependent and IRES-mediated translation. Nucleic Acids Res (2005) 33(9):2962–79. 10.1093/nar/gki603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Coldwell MJ, Mitchell SA, Stoneley M, MacFarlane M, Willis AE. Initiation of Apaf-1 translation by internal ribosome entry. Oncogene (2000) 19(7):899–905. 10.1038/sj.onc.1203407 [DOI] [PubMed] [Google Scholar]
- 93.Liu B, Qian SB. Translational reprogramming in cellular stress response. Wiley Interdiscip Rev RNA (2014) 5(3):301–15. 10.1002/wrna.1212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Li D, Wei T, Abbott CM, Harrich D. The unexpected roles of eukaryotic translation elongation factors in RNA virus replication and pathogenesis. Microbiol Mol Biol Rev (2013) 77(2):253–66. 10.1128/MMBR.00059-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Proud CG. Regulation and roles of elongation factor 2 kinase. Biochem Soc Trans (2015) 43(3):328–32. 10.1042/BST20140323 [DOI] [PubMed] [Google Scholar]
- 96.Ingolia NT, Lareau LF, Weissman JS. Ribosome profiling of mouse embryonic stem cells reveals the complexity and dynamics of mammalian proteomes. Cell (2011) 147(4):789–802. 10.1016/j.cell.2011.10.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Alkalaeva EZ, Pisarev AV, Frolova LY, Kisselev LL, Pestova TV. In vitro reconstitution of eukaryotic translation reveals cooperativity between release factors eRF1 and eRF3. Cell (2006) 125(6):1125–36. 10.1016/j.cell.2006.04.035 [DOI] [PubMed] [Google Scholar]
- 98.Jackson RJ, Hellen CU, Pestova TV. Termination and post-termination events in eukaryotic translation. Adv Protein Chem Struct Biol (2012) 86:45–93. 10.1016/B978-0-12-386497-0.00002-5 [DOI] [PubMed] [Google Scholar]
- 99.Pisarev AV, Skabkin MA, Pisareva VP, Skabkina OV, Rakotondrafara AM, Hentze MW, et al. The role of ABCE1 in eukaryotic posttermination ribosomal recycling. Mol Cell (2010) 37(2):196–210. 10.1016/j.molcel.2009.12.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Young DJ, Guydosh NR, Zhang F, Hinnebusch AG, Green R. Rli1/ABCE1 recycles terminating ribosomes and controls translation reinitiation in 3’UTRs in vivo. Cell (2015) 162(4):872–84. 10.1016/j.cell.2015.07.041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Miettinen TP, Bjorklund M. Modified ribosome profiling reveals high abundance of ribosome protected mRNA fragments derived from 3’ untranslated regions. Nucleic Acids Res (2015) 43(2):1019–34. 10.1093/nar/gku1310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Johnstone TG, Bazzini AA, Giraldez AJ. Upstream ORFs are prevalent translational repressors in vertebrates. EMBO J (2016) 35(7):706–23. 10.15252/embj.201592759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Schleich S, Strassburger K, Janiesch PC, Koledachkina T, Miller KK, Haneke K, et al. DENR-MCT-1 promotes translation re-initiation downstream of uORFs to control tissue growth. Nature (2014) 512(7513):208–12. 10.1038/nature13401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Wolfe AL, Singh K, Zhong Y, Drewe P, Rajasekhar VK, Sanghvi VR, et al. RNA G-quadruplexes cause eIF4A-dependent oncogene translation in cancer. Nature (2014) 513(7516):65–70. 10.1038/nature13485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Jia J, Yao P, Arif A, Fox PL. Regulation and dysregulation of 3’UTR-mediated translational control. Curr Opin Genet Dev (2013) 23(1):29–34. 10.1016/j.gde.2012.12.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Iwakawa HO, Tomari Y. The functions of microRNAs: mRNA decay and translational repression. Trends Cell Biol (2015) 25(11):651–65. 10.1016/j.tcb.2015.07.011 [DOI] [PubMed] [Google Scholar]
- 107.Jonas S, Izaurralde E. Towards a molecular understanding of microRNA-mediated gene silencing. Nat Rev Genet (2015) 16(7):421–33. 10.1038/nrg3965 [DOI] [PubMed] [Google Scholar]
- 108.Baltz AG, Munschauer M, Schwanhausser B, Vasile A, Murakawa Y, Schueler M, et al. The mRNA-bound proteome and its global occupancy profile on protein-coding transcripts. Mol Cell (2012) 46(5):674–90. 10.1016/j.molcel.2012.05.021 [DOI] [PubMed] [Google Scholar]
- 109.Mayr C, Bartel DP. Widespread shortening of 3’UTRs by alternative cleavage and polyadenylation activates oncogenes in cancer cells. Cell (2009) 138(4):673–84. 10.1016/j.cell.2009.06.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Hoffman Y, Bublik DR, Ugalde AP, Elkon R, Biniashvili T, Agami R, et al. 3’UTR shortening potentiates microRNA-based repression of pro-differentiation genes in proliferating human cells. PLoS Genet (2016) 12(2):e1005879. 10.1371/journal.pgen.1005879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Glisovic T, Bachorik JL, Yong J, Dreyfuss G. RNA-binding proteins and post-transcriptional gene regulation. FEBS Lett (2008) 582(14):1977–86. 10.1016/j.febslet.2008.03.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Brummer A, Kishore S, Subasic D, Hengartner M, Zavolan M. Modeling the binding specificity of the RNA-binding protein GLD-1 suggests a function of coding region-located sites in translational repression. RNA (2013) 19(10):1317–26. 10.1261/rna.037531.112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Wurth L, Gebauer F. RNA-binding proteins, multifaceted translational regulators in cancer. Biochim Biophys Acta (2015) 1849(7):881–6. 10.1016/j.bbagrm.2014.10.001 [DOI] [PubMed] [Google Scholar]
- 114.Grammatikakis I, Abdelmohsen K, Gorospe M. Posttranslational control of HuR function. Wiley Interdiscip Rev RNA (2017) 8(1). 10.1002/wrna.1372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Mukherjee N, Corcoran DL, Nusbaum JD, Reid DW, Georgiev S, Hafner M, et al. Integrative regulatory mapping indicates that the RNA-binding protein HuR couples pre-mRNA processing and mRNA stability. Mol Cell (2011) 43(3):327–39. 10.1016/j.molcel.2011.06.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Lebedeva S, Jens M, Theil K, Schwanhausser B, Selbach M, Landthaler M, et al. Transcriptome-wide analysis of regulatory interactions of the RNA-binding protein HuR. Mol Cell (2011) 43(3):340–52. 10.1016/j.molcel.2011.06.008 [DOI] [PubMed] [Google Scholar]
- 117.Srikantan S, Gorospe M. UneCLIPsing HuR nuclear function. Mol Cell (2011) 43(3):319–21. 10.1016/j.molcel.2011.07.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Wang J, Guo Y, Chu H, Guan Y, Bi J, Wang B. Multiple functions of the RNA-binding protein HuR in cancer progression, treatment responses and prognosis. Int J Mol Sci (2013) 14(5):10015–41. 10.3390/ijms140510015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Wang W, Caldwell MC, Lin S, Furneaux H, Gorospe M. HuR regulates cyclin A and cyclin B1 mRNA stability during cell proliferation. EMBO J (2000) 19(10):2340–50. 10.1093/emboj/19.10.2340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Kim HH, Gorospe M. Phosphorylated HuR shuttles in cycles. Cell Cycle (2008) 7(20):3124–6. 10.4161/cc.7.20.6884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Hansen TV, Hammer NA, Nielsen J, Madsen M, Dalbaeck C, Wewer UM, et al. Dwarfism and impaired gut development in insulin-like growth factor II mRNA-binding protein 1-deficient mice. Mol Cell Biol (2004) 24(10):4448–64. 10.1128/MCB.24.10.4448-4464.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Riener MO, Fritzsche FR, Clavien PA, Pestalozzi BC, Probst-Hensch N, Jochum W, et al. IMP3 expression in lesions of the biliary tract: a marker for high-grade dysplasia and an independent prognostic factor in bile duct carcinomas. Hum Pathol (2009) 40(10):1377–83. 10.1016/j.humpath.2009.01.024 [DOI] [PubMed] [Google Scholar]
- 123.Jiang Z, Chu PG, Woda BA, Rock KL, Liu Q, Hsieh CC, et al. Analysis of RNA-binding protein IMP3 to predict metastasis and prognosis of renal-cell carcinoma: a retrospective study. Lancet Oncol (2006) 7(7):556–64. 10.1016/S1470-2045(06)70732-X [DOI] [PubMed] [Google Scholar]
- 124.Jiang Z, Chu PG, Woda BA, Liu Q, Balaji KC, Rock KL, et al. Combination of quantitative IMP3 and tumor stage: a new system to predict metastasis for patients with localized renal cell carcinomas. Clin Cancer Res (2008) 14(17):5579–84. 10.1158/1078-0432.CCR-08-0504 [DOI] [PubMed] [Google Scholar]
- 125.Sitnikova L, Mendese G, Liu Q, Woda BA, Lu D, Dresser K, et al. IMP3 predicts aggressive superficial urothelial carcinoma of the bladder. Clin Cancer Res (2008) 14(6):1701–6. 10.1158/1078-0432.CCR-07-2039 [DOI] [PubMed] [Google Scholar]
- 126.Bell JL, Wachter K, Muhleck B, Pazaitis N, Kohn M, Lederer M, et al. Insulin-like growth factor 2 mRNA-binding proteins (IGF2BPs): post-transcriptional drivers of cancer progression? Cell Mol Life Sci (2013) 70(15):2657–75. 10.1007/s00018-012-1186-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Bernstein PL, Herrick DJ, Prokipcak RD, Ross J. Control of c-myc mRNA half-life in vitro by a protein capable of binding to a coding region stability determinant. Genes Dev (1992) 6(4):642–54. 10.1101/gad.6.4.642 [DOI] [PubMed] [Google Scholar]
- 128.Faye MD, Beug ST, Graber TE, Earl N, Xiang X, Wild B, et al. IGF2BP1 controls cell death and drug resistance in rhabdomyosarcomas by regulating translation of cIAP1. Oncogene (2015) 34(12):1532–41. 10.1038/onc.2014.90 [DOI] [PubMed] [Google Scholar]
- 129.Lederer M, Bley N, Schleifer C, Huttelmaier S. The role of the oncofetal IGF2 mRNA-binding protein 3 (IGF2BP3) in cancer. Semin Cancer Biol (2014) 29:3–12. 10.1016/j.semcancer.2014.07.006 [DOI] [PubMed] [Google Scholar]
- 130.Galardi S, Petretich M, Pinna G, D’Amico S, Loreni F, Michienzi A, et al. CPEB1 restrains proliferation of glioblastoma cells through the regulation of p27(Kip1) mRNA translation. Sci Rep (2016) 6:25219. 10.1038/srep25219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Richter JD. CPEB: a life in translation. Trends Biochem Sci (2007) 32(6):279–85. 10.1016/j.tibs.2007.04.004 [DOI] [PubMed] [Google Scholar]
- 132.Grudzien-Nogalska E, Reed BC, Rhoads RE. CPEB1 promotes differentiation and suppresses EMT in mammary epithelial cells. J Cell Sci (2014) 127(Pt 10):2326–38. 10.1242/jcs.144956 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Nagaoka K, Fujii K, Zhang H, Usuda K, Watanabe G, Ivshina M, et al. CPEB1 mediates epithelial-to-mesenchyme transition and breast cancer metastasis. Oncogene (2016) 35(22):2893–901. 10.1038/onc.2015.350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Ortiz-Zapater E, Pineda D, Martinez-Bosch N, Fernandez-Miranda G, Iglesias M, Alameda F, et al. Key contribution of CPEB4-mediated translational control to cancer progression. Nat Med (2011) 18(1):83–90. 10.1038/nm.2540 [DOI] [PubMed] [Google Scholar]
- 135.Perez-Guijarro E, Karras P, Cifdaloz M, Martinez-Herranz R, Canon E, Grana O, et al. Lineage-specific roles of the cytoplasmic polyadenylation factor CPEB4 in the regulation of melanoma drivers. Nat Commun (2016) 7:13418. 10.1038/ncomms13418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Meyer KD, Jaffrey SR. The dynamic epitranscriptome: N6-methyladenosine and gene expression control. Nat Rev Mol Cell Biol (2014) 15(5):313–26. 10.1038/nrm3785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Gilbert WV, Bell TA, Schaening C. Messenger RNA modifications: form, distribution, and function. Science (2016) 352(6292):1408–12. 10.1126/science.aad8711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Li X, Xiong X, Yi C. Epitranscriptome sequencing technologies: decoding RNA modifications. Nat Methods (2016) 14(1):23–31. 10.1038/nmeth.4110 [DOI] [PubMed] [Google Scholar]
- 139.Iles MM, Law MH, Stacey SN, Han J, Fang S, Pfeiffer R, et al. A variant in FTO shows association with melanoma risk not due to BMI. Nat Genet (2013) 45(4):428–32, 432e1. 10.1038/ng.2571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Garcia-Closas M, Couch FJ, Lindstrom S, Michailidou K, Schmidt MK, Brook MN, et al. Genome-wide association studies identify four ER negative-specific breast cancer risk loci. Nat Genet (2013) 45(4):392–8, 8e1–2. 10.1038/ng.2561 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Meyer KD, Saletore Y, Zumbo P, Elemento O, Mason CE, Jaffrey SR. Comprehensive analysis of mRNA methylation reveals enrichment in 3’ UTRs and near stop codons. Cell (2012) 149(7):1635–46. 10.1016/j.cell.2012.05.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Molinie B, Wang J, Lim KS, Hillebrand R, Lu ZX, Van Wittenberghe N, et al. m(6)A-LAIC-seq reveals the census and complexity of the m(6)A epitranscriptome. Nat Methods (2016) 13(8):692–8. 10.1038/nmeth.3898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Meyer KD, Patil DP, Zhou J, Zinoviev A, Skabkin MA, Elemento O, et al. 5’ UTR m(6)A promotes cap-independent translation. Cell (2015) 163(4):999–1010. 10.1016/j.cell.2015.10.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Zhou J, Wan J, Gao X, Zhang X, Jaffrey SR, Qian SB. Dynamic m(6)A mRNA methylation directs translational control of heat shock response. Nature (2015) 526(7574):591–4. 10.1038/nature15377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Jiang Q, Crews LA, Holm F, Jamieson CHM. RNA editing-dependent epitranscriptome diversity in cancer stem cells. Nat Rev Cancer (2017) 17(6):381–92. 10.1038/nrc.2017.23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Bahn JH, Ahn J, Lin X, Zhang Q, Lee JH, Civelek M, et al. Genomic analysis of ADAR1 binding and its involvement in multiple RNA processing pathways. Nat Commun (2015) 6:6355. 10.1038/ncomms7355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Barna M, Pusic A, Zollo O, Costa M, Kondrashov N, Rego E, et al. Suppression of Myc oncogenic activity by ribosomal protein haploinsufficiency. Nature (2008) 456(7224):971–5. 10.1038/nature07449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Truitt ML, Conn CS, Shi Z, Pang X, Tokuyasu T, Coady AM, et al. Differential requirements for eIF4E dose in normal development and cancer. Cell (2015) 162(1):59–71. 10.1016/j.cell.2015.05.049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Shahbazian D, Parsyan A, Petroulakis E, Hershey J, Sonenberg N. eIF4B controls survival and proliferation and is regulated by proto-oncogenic signaling pathways. Cell Cycle (2010) 9(20):4106–9. 10.4161/cc.9.20.13630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Lazaris-Karatzas A, Montine KS, Sonenberg N. Malignant transformation by a eukaryotic initiation factor subunit that binds to mRNA 5’ cap. Nature (1990) 345(6275):544–7. 10.1038/345544a0 [DOI] [PubMed] [Google Scholar]
- 151.Mamane Y, Petroulakis E, Rong L, Yoshida K, Ler LW, Sonenberg N. eIF4E – from translation to transformation. Oncogene (2004) 23(18):3172–9. 10.1038/sj.onc.1207549 [DOI] [PubMed] [Google Scholar]
- 152.Yanagiya A, Suyama E, Adachi H, Svitkin YV, Aza-Blanc P, Imataka H, et al. Translational homeostasis via the mRNA cap-binding protein, eIF4E. Mol Cell (2012) 46(6):847–58. 10.1016/j.molcel.2012.04.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Koromilas AE, Lazaris-Karatzas A, Sonenberg N. mRNAs containing extensive secondary structure in their 5’ non-coding region translate efficiently in cells overexpressing initiation factor eIF-4E. EMBO J (1992) 11(11):4153–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Niedzwiecka A, Marcotrigiano J, Stepinski J, Jankowska-Anyszka M, Wyslouch-Cieszynska A, Dadlez M, et al. Biophysical studies of eIF4E cap-binding protein: recognition of mRNA 5’ cap structure and synthetic fragments of eIF4G and 4E-BP1 proteins. J Mol Biol (2002) 319(3):615–35. 10.1016/S0022-2836(02)00328-5 [DOI] [PubMed] [Google Scholar]
- 155.Topisirovic I, Culjkovic B, Cohen N, Perez JM, Skrabanek L, Borden KL. The proline-rich homeodomain protein, PRH, is a tissue-specific inhibitor of eIF4E-dependent cyclin D1 mRNA transport and growth. EMBO J (2003) 22(3):689–703. 10.1093/emboj/cdg069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Ueda T, Watanabe-Fukunaga R, Fukuyama H, Nagata S, Fukunaga R. Mnk2 and Mnk1 are essential for constitutive and inducible phosphorylation of eukaryotic initiation factor 4E but not for cell growth or development. Mol Cell Biol (2004) 24(15):6539–49. 10.1128/MCB.24.15.6539-6549.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Scheper GC, van Kollenburg B, Hu J, Luo Y, Goss DJ, Proud CG. Phosphorylation of eukaryotic initiation factor 4E markedly reduces its affinity for capped mRNA. J Biol Chem (2002) 277(5):3303–9. 10.1074/jbc.M103607200 [DOI] [PubMed] [Google Scholar]
- 158.Zuberek J, Wyslouch-Cieszynska A, Niedzwiecka A, Dadlez M, Stepinski J, Augustyniak W, et al. Phosphorylation of eIF4E attenuates its interaction with mRNA 5’ cap analogs by electrostatic repulsion: intein-mediated protein ligation strategy to obtain phosphorylated protein. RNA (2003) 9(1):52–61. 10.1261/rna.2133403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Pettersson F, Del Rincon SV, Emond A, Huor B, Ngan E, Ng J, et al. Genetic and pharmacologic inhibition of eIF4E reduces breast cancer cell migration, invasion, and metastasis. Cancer Res (2015) 75(6):1102–12. 10.1158/0008-5472.CAN-14-1996 [DOI] [PubMed] [Google Scholar]
- 160.Robichaud N, del Rincon SV, Huor B, Alain T, Petruccelli LA, Hearnden J, et al. Phosphorylation of eIF4E promotes EMT and metastasis via translational control of SNAIL and MMP-3. Oncogene (2015) 34(16):2032–42. 10.1038/onc.2014.146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Xie J, Wang X, Proud CG. mTOR inhibitors in cancer therapy. F1000Res (2016) 5:1–11. 10.12688/f1000research.9207.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Gandin V, Masvidal L, Cargnello M, Gyenis L, McLaughlan S, Cai Y, et al. mTORC1 and CK2 coordinate ternary and eIF4F complex assembly. Nat Commun (2016) 7:11127. 10.1038/ncomms11127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Roux PP, Topisirovic I. Regulation of mRNA translation by signaling pathways. Cold Spring Harb Perspect Biol (2012) 4(11):a012252. 10.1101/cshperspect.a012252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Alain T, Morita M, Fonseca BD, Yanagiya A, Siddiqui N, Bhat M, et al. eIF4E/4E-BP ratio predicts the efficacy of mTOR targeted therapies. Cancer Res (2012) 72(24):6468–76. 10.1158/0008-5472.CAN-12-2395 [DOI] [PubMed] [Google Scholar]
- 165.Tsukumo Y, Alain T, Fonseca BD, Nadon R, Sonenberg N. Translation control during prolonged mTORC1 inhibition mediated by 4E-BP3. Nat Commun (2016) 7:11776. 10.1038/ncomms11776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Raught B, Peiretti F, Gingras AC, Livingstone M, Shahbazian D, Mayeur GL, et al. Phosphorylation of eucaryotic translation initiation factor 4B Ser422 is modulated by S6 kinases. EMBO J (2004) 23(8):1761–9. 10.1038/sj.emboj.7600193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Shahbazian D, Roux PP, Mieulet V, Cohen MS, Raught B, Taunton J, et al. The mTOR/PI3K and MAPK pathways converge on eIF4B to control its phosphorylation and activity. EMBO J (2006) 25(12):2781–91. 10.1038/sj.emboj.7601166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Shahbazian D, Parsyan A, Petroulakis E, Topisirovic I, Martineau Y, Gibbs BF, et al. Control of cell survival and proliferation by mammalian eukaryotic initiation factor 4B. Mol Cell Biol (2010) 30(6):1478–85. 10.1128/MCB.01218-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Yang HS, Jansen AP, Komar AA, Zheng X, Merrick WC, Costes S, et al. The transformation suppressor Pdcd4 is a novel eukaryotic translation initiation factor 4A binding protein that inhibits translation. Mol Cell Biol (2003) 23(1):26–37. 10.1128/MCB.23.1.26-37.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Mayer C, Zhao J, Yuan X, Grummt I. mTOR-dependent activation of the transcription factor TIF-IA links rRNA synthesis to nutrient availability. Genes Dev (2004) 18(4):423–34. 10.1101/gad.285504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Michels AA, Robitaille AM, Buczynski-Ruchonnet D, Hodroj W, Reina JH, Hall MN, et al. mTORC1 directly phosphorylates and regulates human MAF1. Mol Cell Biol (2010) 30(15):3749–57. 10.1128/MCB.00319-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Wei Y, Tsang CK, Zheng XF. Mechanisms of regulation of RNA polymerase III-dependent transcription by TORC1. EMBO J (2009) 28(15):2220–30. 10.1038/emboj.2009.179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Fernandez PC, Frank SR, Wang L, Schroeder M, Liu S, Greene J, et al. Genomic targets of the human c-Myc protein. Genes Dev (2003) 17(9):1115–29. 10.1101/gad.1067003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Lin CJ, Malina A, Pelletier J. c-Myc and eIF4F constitute a feedforward loop that regulates cell growth: implications for anticancer therapy. Cancer Res (2009) 69(19):7491–4. 10.1158/0008-5472.CAN-09-0813 [DOI] [PubMed] [Google Scholar]
- 175.Lin CJ, Cencic R, Mills JR, Robert F, Pelletier J. c-Myc and eIF4F are components of a feedforward loop that links transcription and translation. Cancer Res (2008) 68(13):5326–34. 10.1158/0008-5472.CAN-07-5876 [DOI] [PubMed] [Google Scholar]
- 176.Khanna-Gupta A, Abayasekara N, Levine M, Sun H, Virgilio M, Nia N, et al. Up-regulation of translation eukaryotic initiation factor 4E in nucleophosmin 1 haploinsufficient cells results in changes in CCAAT enhancer-binding protein alpha activity: implications in myelodysplastic syndrome and acute myeloid leukemia. J Biol Chem (2012) 287(39):32728–37. 10.1074/jbc.M112.373274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Hariri F, Arguello M, Volpon L, Culjkovic-Kraljacic B, Nielsen TH, Hiscott J, et al. The eukaryotic translation initiation factor eIF4E is a direct transcriptional target of NF-kappaB and is aberrantly regulated in acute myeloid leukemia. Leukemia (2013) 27(10):2047–55. 10.1038/leu.2013.73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Yi T, Papadopoulos E, Hagner PR, Wagner G. Hypoxia-inducible factor-1alpha (HIF-1alpha) promotes cap-dependent translation of selective mRNAs through up-regulating initiation factor eIF4E1 in breast cancer cells under hypoxia conditions. J Biol Chem (2013) 288(26):18732–42. 10.1074/jbc.M113.471466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Topisirovic I, Siddiqui N, Orolicki S, Skrabanek LA, Tremblay M, Hoang T, et al. Stability of eukaryotic translation initiation factor 4E mRNA is regulated by HuR, and this activity is dysregulated in cancer. Mol Cell Biol (2009) 29(5):1152–62. 10.1128/MCB.01532-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Koromilas AE. Roles of the translation initiation factor eIF2alpha serine 51 phosphorylation in cancer formation and treatment. Biochim Biophys Acta (2015) 1849(7):871–80. 10.1016/j.bbagrm.2014.12.007 [DOI] [PubMed] [Google Scholar]
- 181.Holcik M. Could the eIF2alpha-independent translation be the Achilles heel of cancer? Front Oncol (2015) 5:264. 10.3389/fonc.2015.00264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Vattem KM, Wek RC. Reinitiation involving upstream ORFs regulates ATF4 mRNA translation in mammalian cells. Proc Natl Acad Sci U S A (2004) 101(31):11269–74. 10.1073/pnas.0400541101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Somers J, Wilson LA, Kilday JP, Horvilleur E, Cannell IG, Poyry TA, et al. A common polymorphism in the 5’ UTR of ERCC5 creates an upstream ORF that confers resistance to platinum-based chemotherapy. Genes Dev (2015) 29(18):1891–6. 10.1101/gad.261867.115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Richter JD, Coller J. Pausing on polyribosomes: make way for elongation in translational control. Cell (2015) 163(2):292–300. 10.1016/j.cell.2015.09.041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Reid DW, Nicchitta CV. Diversity and selectivity in mRNA translation on the endoplasmic reticulum. Nat Rev Mol Cell Biol (2015) 16(4):221–31. 10.1038/nrm3958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Jagannathan S, Hsu JC, Reid DW, Chen Q, Thompson WJ, Moseley AM, et al. Multifunctional roles for the protein translocation machinery in RNA anchoring to the endoplasmic reticulum. J Biol Chem (2014) 289(37):25907–24. 10.1074/jbc.M114.580688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Reid DW, Nicchitta CV. Primary role for endoplasmic reticulum-bound ribosomes in cellular translation identified by ribosome profiling. J Biol Chem (2012) 287(8):5518–27. 10.1074/jbc.M111.312280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Chaudhury A, Hussey GS, Ray PS, Jin G, Fox PL, Howe PH. TGF-beta-mediated phosphorylation of hnRNP E1 induces EMT via transcript-selective translational induction of Dab2 and ILEI. Nat Cell Biol (2010) 12(3):286–93. 10.1038/ncb2029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Hussey GS, Chaudhury A, Dawson AE, Lindner DJ, Knudsen CR, Wilce MC, et al. Identification of an mRNP complex regulating tumorigenesis at the translational elongation step. Mol Cell (2011) 41(4):419–31. 10.1016/j.molcel.2011.02.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Ryazanov AG, Shestakova EA, Natapov PG. Phosphorylation of elongation factor 2 by EF-2 kinase affects rate of translation. Nature (1988) 334(6178):170–3. 10.1038/334170a0 [DOI] [PubMed] [Google Scholar]
- 191.Faller WJ, Jackson TJ, Knight JR, Ridgway RA, Jamieson T, Karim SA, et al. mTORC1-mediated translational elongation limits intestinal tumour initiation and growth. Nature (2015) 517(7535):497–500. 10.1038/nature13896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Liu JC, Voisin V, Wang S, Wang DY, Jones RA, Datti A, et al. Combined deletion of Pten and p53 in mammary epithelium accelerates triple-negative breast cancer with dependency on eEF2K. EMBO Mol Med (2014) 6(12):1542–60. 10.15252/emmm.201404402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Xue S, Barna M. Specialized ribosomes: a new frontier in gene regulation and organismal biology. Nat Rev Mol Cell Biol (2012) 13(6):355–69. 10.1038/nrm3359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Truitt ML, Ruggero D. New frontiers in translational control of the cancer genome. Nat Rev Cancer (2016) 16(5):288–304. 10.1038/nrc.2016.27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Novoa EM, Ribas de Pouplana L. Speeding with control: codon usage, tRNAs, and ribosomes. Trends Genet (2012) 28(11):574–81. 10.1016/j.tig.2012.07.006 [DOI] [PubMed] [Google Scholar]
- 196.Pavon-Eternod M, Gomes S, Rosner MR, Pan T. Overexpression of initiator methionine tRNA leads to global reprogramming of tRNA expression and increased proliferation in human epithelial cells. RNA (2013) 19(4):461–6. 10.1261/rna.037507.112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Felton-Edkins ZA, Kenneth NS, Brown TR, Daly NL, Gomez-Roman N, Grandori C, et al. Direct regulation of RNA polymerase III transcription by RB, p53 and c-Myc. Cell Cycle (2003) 2(3):181–4. 10.4161/cc.2.3.375 [DOI] [PubMed] [Google Scholar]
- 198.Felton-Edkins ZA, Fairley JA, Graham EL, Johnston IM, White RJ, Scott PH. The mitogen-activated protein (MAP) kinase ERK induces tRNA synthesis by phosphorylating TFIIIB. EMBO J (2003) 22(10):2422–32. 10.1093/emboj/cdg240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Gingold H, Tehler D, Christoffersen NR, Nielsen MM, Asmar F, Kooistra SM, et al. A dual program for translation regulation in cellular proliferation and differentiation. Cell (2014) 158(6):1281–92. 10.1016/j.cell.2014.08.011 [DOI] [PubMed] [Google Scholar]
- 200.Pavon-Eternod M, Gomes S, Geslain R, Dai Q, Rosner MR, Pan T. tRNA over-expression in breast cancer and functional consequences. Nucleic Acids Res (2009) 37(21):7268–80. 10.1093/nar/gkp787 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Grewal SS. Why should cancer biologists care about tRNAs? tRNA synthesis, mRNA translation and the control of growth. Biochim Biophys Acta (2015) 1849(7):898–907. 10.1016/j.bbagrm.2014.12.005 [DOI] [PubMed] [Google Scholar]
- 202.Gu C, Begley TJ, Dedon PC. tRNA modifications regulate translation during cellular stress. FEBS Lett (2014) 588(23):4287–96. 10.1016/j.febslet.2014.09.038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Park SG, Schimmel P, Kim S. Aminoacyl tRNA synthetases and their connections to disease. Proc Natl Acad Sci U S A (2008) 105(32):11043–9. 10.1073/pnas.0802862105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Jia J, Arif A, Ray PS, Fox PL. WHEP domains direct noncanonical function of glutamyl-prolyl tRNA synthetase in translational control of gene expression. Mol Cell (2008) 29(6):679–90. 10.1016/j.molcel.2008.01.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.McCune SA, Morris HP, Weber G. Neoplastic transformation-linked alterations in arginyl-tRNA synthetase activity. Biochim Biophys Acta (1979) 561(2):410–20. 10.1016/0005-2787(79)90149-7 [DOI] [PubMed] [Google Scholar]
- 206.Thomas K, Scheets K, Allen S, Hedgcoth C. Comparison of complexes containing lysyl-tRNA synthetase from normal and virus-transformed cells. Can J Biochem (1982) 60(8):804–10. 10.1139/o82-100 [DOI] [PubMed] [Google Scholar]
- 207.Kim S, You S, Hwang D. Aminoacyl-tRNA synthetases and tumorigenesis: more than housekeeping. Nat Rev Cancer (2011) 11(10):708–18. 10.1038/nrc3124 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.