Abstract
A novel set of integrated procedures for quantification of fibroblast‐rich stroma and vascular characteristics has recently been presented allowing discovery of novel perivascular and stromal biomarkers in colorectal, renal cell, and ovarian cancer. In the present study, data obtained through these procedures from clinically well‐annotated collections of these three tumour types have been used to address two novel questions. First, data have been used to investigate if the three tumour types demonstrate significant differences regarding features such as vessel diameter, vessel density, and perivascular marker expression. Second, analyses of the cohorts have been used to explore the prognostic significance of a novel vascular metric, ‘vessel distance inter‐quartile range (IQR)’ that describes intra‐case heterogeneity regarding vessel distribution. The comparisons between the three tumour types demonstrated a set of significant differences. Vessel density of renal cell cancer was statistically significantly higher than in colorectal and ovarian cancer. Vessel diameter was statistically significantly higher in ovarian cancer. Concerning perivascular status, colorectal cancer displayed significantly higher levels of perivascular PDGFR‐β expression than the other two tumour types. Intra‐case heterogeneity of perivascular PDGFR‐β expression was also higher in colorectal cancer. Notably, these fibroblast‐dominated stroma phenotypes matched previously described experimental tumour stroma characteristics, which have been linked to differential sensitivity to anti‐VEGF drugs. High ‘vessel distance IQR’ was significantly associated with poor survival in both renal cell cancer and colorectal cancer. In renal cell cancer, this characteristic also acted as an independent prognostic marker according to multivariate analyses including standard clinico‐pathological characteristics. Explorative subset analyses indicated particularly strong prognostic significance of ‘vessel distance IQR’ in T stage 4 of this cancer type. Together, these analyses identified tumour‐type‐specific vascular‐stroma phenotypes of possible functional significance, and suggest ‘vessel distance IQR’ as a novel prognostic biomarker.
Keywords: colorectal cancer, renal cell cancer, ovarian cancer, tumour microenvironment, vessels, pericytes, PDGFR‐β, survival
Introduction
Tumour vasculature impacts on multiple aspects of tumour biology 1, 2. The vasculature of solid tumours affects growth and metastasis propensity. Interactions between vascular cells and immune cells also impact on immune surveillance. Furthermore, vascular properties regulate chemotherapy sensitivity by affecting drug delivery.
Analyses of properties such as vessel density, vessel maturation, endothelial cell proliferation, marker expression, and pericyte status have demonstrated that these features are associated with prognosis in many tumour types, such as breast, prostate, ovarian, clear cell renal cell carcinomas, and glioblastoma 2, 3, 4, 5, 6.
Anti‐angiogenic treatments targeting VEGF receptor signaling have been tested in many different tumour types for treatment of metastatic disease or in adjuvant settings 7, 8. Results from these clinical studies have demonstrated tumour‐type‐specific differences in sensitivity to these drugs when used as mono‐treatments. Well‐known examples of tumour‐type‐specific differences in efficacy include, for example, the robust effects of mono‐treatment with anti‐angiogenic drugs in renal cell cancer 9, the modest effects in ovarian cancer 10, and the lack of effects in colorectal cancer 11.
These differences in sensitivity to anti‐angiogenic drugs could reflect systematic functional and structural differences in the tumour vasculature of different tumour types. Such differences could be caused by organ‐specific differences in physiological vascular biology programme. It can also be envisioned that tumour‐type‐specific oncogenic drivers are initiating different angiogenic programmes. Furthermore, results from studies of metastatic lesions show that different tumour types use angiogenesis or vessel co‐option to varying degrees, presenting vasculatures with different degree of maturation 12, 13. Systematic comparisons of vascular biology features between different tumour types therefore have the potential to uncover tumour‐type‐specific features of vascular biology with mechanistic or therapeutic potential.
Most prognosis studies with vascular markers have used case‐based mean‐values of the marker‐of‐interest as the basis for scoring of individual tumours. Recent analyses in ovarian and kidney cancer have demonstrated that the inside‐case heterogeneity regarding expression of a perivascular marker acts as a prognostic factor 14. This suggests the possibility of prognostic significance also of heterogeneity of other tumour vasculature characteristics.
This study exploits a set of integrated procedures for quantification of vascular characteristics, which recently uncovered novel vascular biomarkers and suggested novel biological mechanisms in ovarian, renal cell, and colorectal cancer 5, 14, 15. In this study, data from four tumour collections (colorectal cancer cohort, colon cancer cohort, ovarian cancer, and kidney cancer cohorts) have been used to compare vascular characteristics between tumour types. Furthermore, the previous demonstration of prognostic significance of perivascular heterogeneity has been extended to analyses of the potential prognostic significance of ‘vessel distance IQR’; a ‘metric’ capturing intra‐case heterogeneity regarding inter‐vessel distances 14.
Material and methods
Tumour cohorts and tissue micro‐arrays
Details about the tumour cohorts have been described previously 5, 14, 16. In brief, the ovarian cancer collection (OC) is composed of 109 cases of primary adnexal site tissues deriving from patients affected by serous ovarian cancer collected consecutively between 1985 and 2006 at the University Medical center Groningen (Groningen, The Netherlands) 17. The renal cell cancer collection (RCC) is composed of 288 primary tumours from surgically treated patients diagnosed at Malmo University Hospital (current Skåne University Hospital), between 1978 and 1996 14. The colorectal cancer collection (CRC) is composed of primary tumours from 447 patients diagnosed in Malmo University Hospital. This cohort originates from the population‐based prospective study, the Malmo Diet and Cancer Cohort, that enrolled individuals between 1991 and 1996 16. The colon cancer cohort was made up of primary tissues derived from 93 patients from a Nordic randomized clinical trial performed to evaluate the efficacy of 5‐FU (5‐fluorouracil)‐based adjuvant chemotherapy 18. The original study included 2224 patients younger than 76 years with stages II‐III colon cancer radically resected in 1991 – 1997. The present study is based on a selection from the original study of Swedish cases of colon cancer. No patient received radiotherapy or chemotherapy prior to surgery.
All tissues were collected prior to chemotherapy treatment. Preparation of tissue micro‐arrays followed standard procedures and is described in previous publications 5, 14, 16, 17. In the different TMAs, each case is represented by two (colorectal and kidney) or up to four (ovarian cancer) ‘tissue punches’. TMA preparation was approved by appropriate ethical committees.
Ethical approvals have been obtained (Dnr 530/2008 for the colorectal collection, Malmo Diet and Cancer Cohort; Dnr 00–260, 2014/664–32 for the Mammoth CRC‐cohort, Dnr 282/2007 for the renal cell cancer collection Skåne University Hospital; Dnr 2016/551–32 for the ovarian cancer collection, original certification can be provided by Dr G. Bea A. Wisman).
Immunohistochemistry and slide scanning
Procedures for double staining with PDGFR‐β/α‐SMA and CD34 antibodies have been described earlier together with details of the α‐SMA detection antibody 5, 14, 15. The antibody used for PDGFR‐β detection was 28E1 Rabbit mAb, 3169, Cell Signaling Technology. CD34 stains were performed with the antibody clone QBEnd 10, M7165 Mouse mAb, DAKO 5, 14, 15. Detection of primary antibodies followed standard procedures as described previously. Stained slides were subsequently scanned using a Vslide scanning microscope (Metasystems, Alltlussheim, Germany).
Specificity of the PDGF‐β‐receptor antibody has been extensively characterized in earlier studies, including test‐staining of sections of paraformaldehyde‐fixed paraffin‐embedded cultured cells of known PDGF receptor status 19.
Images analyses
Images were analyzed with ImageJ software using an algorithm developed in‐house 14, 15. Subsequent data analysis was performed with R (R 3.2.2 GUI 1.66 Mavericks build (6996) http://www.R-project.org).
CD34 staining was used to determine vessel density, vessel diameter, and vessel distance to the nearest vessel. α‐SMA‐ and PDGFR‐β‐staining were used as markers for cancer‐associated fibroblasts and perivascular cells.
Perivascular area was defined as the region circumjacent to, and extending 5.5 µm away from, the vessel. The intensity of perivascular marker expression (α‐SMA or PDGFR‐β) was measured in each such area. A case‐based value was obtained (perivascular intensity (PVI) by calculating the median intensity for all perivascular areas of each case (see Frödin et al for more details 14).
Marker‐positive stroma fraction was defined as the fraction of the analyzed sample, excluding perivascular regions (see above) and vessel regions, that displayed an expression level above a threshold level. (see Frodin et al for more details 14).
For scoring of PDGFR‐β intensity, we used a tumour‐type‐specific scale to accommodate potential systematic differences between the cohorts regarding, for example, tissue quality and antigen preservation. The intensity was determined within each tumour type using a scale ranging from 0 to 1 with 0 as the lowest noted intensity and 1 as the highest detected intensity. This enabled comparison between tumour types regarding how cases were distributed within each tumour‐specific scale.
A set of procedures was used to obtain a value for each case that would be related to vessel density heterogeneity. First, distance to closest vessel was determined for all vessels of all cases. Second, a median‐based value for vessel‐distance was determined for each case. Third, to quantitate the intra‐case heterogeneity of vessel distribution, the difference between first and third quartiles were calculated for each case. This metric was designated ‘vessel distance IQR’.
Data processing
For comparison between tumour types for marker‐intensity‐related metrics, the original data for each tumour type was normalized and given values between 0 and 1. Normalization was performed case‐wise for fibroblast‐dominated stroma intensity and vessel‐wise for perivascular status. Normalization addressed the issue of skewed distribution of data by skewness adjustment as described by Vanderviere 20 and ‘vessel distance IQR’ metrics were dichotomized per the median.
Statistical analyses
All tests were carried out at the 95% statistical significance level and were performed using SPSS versions 22 and 23 (SPSS Inc., Chicago, IL) and Rstudio (Version 0.99.489 – © 2009–2015 RStudio, Inc.)
Differences between tumour types regarding case‐based values for fibroblast‐dominated stroma metrics, as analyzed in in Figure 1, were determined using the Mann‐Whitney U test. Correlations between case‐based stroma metrics, as analyzed in Figure 2, were determined using the Spearman correlation test for pair‐wise analyses. Log Rank Tests and Cox Regression Models were used to estimate relationships between analyzed metrics and overall survival (Figure 4, Table 1, and supplementary material, Table S3). Associations with the clinico‐pathological characteristics were evaluated with Pearson's Chi Square test (supplementary material, Table S2a–c).
Figure 1.
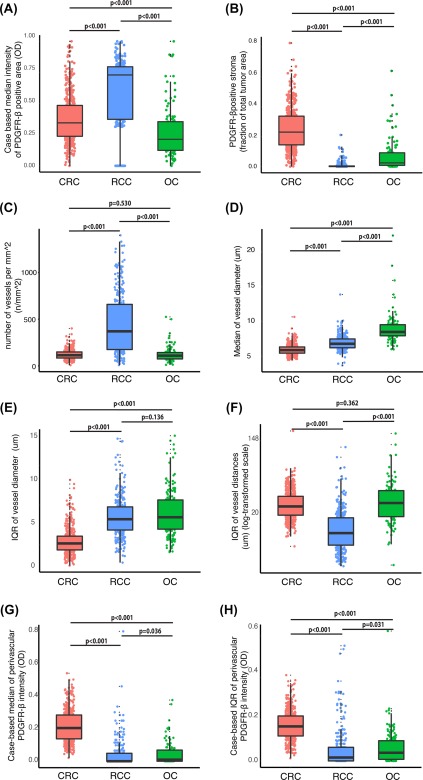
Comparisons of CRC, RCC, and OC regarding case‐based values for vascular and stroma features. Box plots comparing CRC, RCC, and OC tumours with regard to case‐based values for stroma features. The tumour stroma features analyzed are (A) mean intensity of stromal PDGFR‐β‐expression; (B) abundance of PDGFR‐β‐positive tumour stroma; (C) vessel density; (D) mean vessel size; (E) vessel size heterogeneity; (F) heterogeneity regarding inter‐vessel distances, ‘vessel distance IQR’; (G) mean intensity of perivascular PDGFR‐β‐expression; and (H) heterogeneity regarding perivascular PDGFR‐β‐expression. For procedures used to obtained case‐based values for these stroma features, see Material and Methods section.
Figure 2.
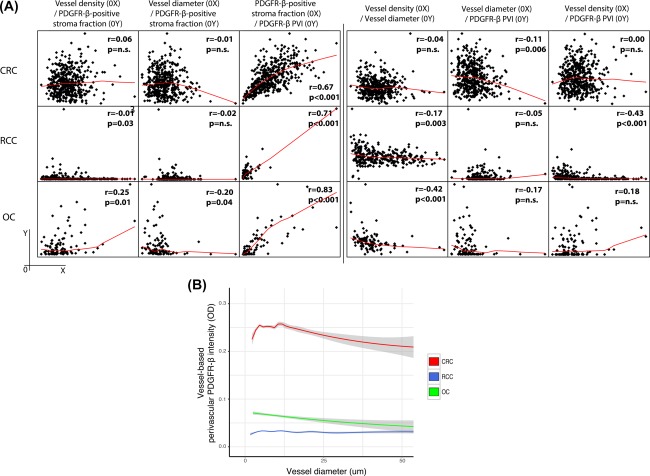
Comparisons of CRC, RCC, and OC regarding correlations between different stroma features. (A) Panels illustrate pair‐wise correlation analyses of case‐based values for stroma features in CRC (upper), RCC (middle), and OC (lower). The left part contains analyses of the correlations between vascular features (vessel density, vessel diameter, and mean intensity of perivascular PDGFR‐β‐expression [PDGFR‐β PVI]) and abundance of PDGFR‐β‐positive tumour stroma (PDGFR‐β‐positive stroma fraction). The right part contains analyses of the correlations between the three different vascular features. For procedures used to obtained case‐based values for these stroma features, see Material and Methods section. (B) The graph illustrates the relationship between vessel diameter and perivascular PDGFR‐β‐intensity in vessels of CRC, RCC, and OC using vessel‐based values. For details concerning methods to obtain data for perivascular PDGFR‐β‐status and diameter of individual vessels, see Material and Methods.
Figure 4.
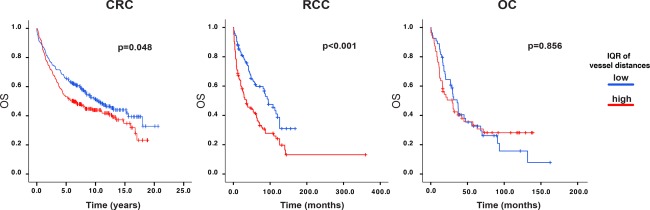
Associations between heterogeneity of inter‐vessel‐distances and overall survival in CRC, RCC, and OC. Kaplan‐Meier curves for overall survival, with P values of Log Rank test, in CRC, RCC, and OC for cases defined by vessel distance IQR.
Table 1.
Uni‐ and multivariate Cox regression analyses of survival association of ‘vessel distance IQR’ and clinical characteristics in RCC
| Variables | Univariate analysis | Multivariate analysis | ||
|---|---|---|---|---|
| HR (95% CI) | P value | HR (95% CI) | P value | |
| Age at diagnosis | ||||
| <50 | 1 (reference) | 0.01 | 1 (reference) | 0.03 |
| >50 | 2.11(1.17–3.81) | 2.94 (1.08–8.00) | ||
| Gender | ||||
| Female 0 | 1 (reference) | 0.80 | 1 (reference) | 0.82 |
| Male 1 | 1.04 (0.76–1.42) | 0.93 (0.50–1.72) | ||
| T stage | ||||
| 1 | 1 (reference) | 0.11 | 1 (reference) | 0.36 |
| 2 | 2.56 (0.82–8.06) | 0.035 | 2.15 (0.43–10.82) | 0.05 |
| 3 | 3.38 (1.09–10.48) | < 0.001 | 4.63 (0.99–21.74) | 0.003 |
| 4 | 12.1 (4.31–33.99) | 9.87 (2.16–45.20) | ||
| M stage | ||||
| 0 | 1 (reference) | <0.001 | 1 (reference) | 0.002 |
| 1 | 6.22 (4.29–9.02) | 3.6 (1.60–8.10) | ||
| Fuhrman grade | ||||
| 1 | 1 (reference) | 0.53 | 1 (reference) | 0.76 |
| 2 | 1.13 (0.71–1.51) | <0.001 | 1.14 (0.48–2.69) | 0.21 |
| 3 | 2.49 (1.57–3.50) | <0.001 | 1.76 (0.72–4.26) | 0.12 |
| 4 | 8.6 (5.12–14.84) | 2.61 (0.78–8.80) | ||
| Histology | ||||
| Non‐clear cell | 1 (reference) | 0.73 | 1 (reference) | 0.53 |
| Clear cell | 1.14 (0.56–2.33) | 1.44 (0.47–4.47) | ||
| IQR vessel distances | ||||
| Low | 1 (reference) | <0.001 | 1 (reference) | 0.006 |
| High | 1.95 (1.42–2.68) | 2.46 (1.29–4.69) | ||
Results
Comparison between tumour types with regard to fibroblast‐dominated stroma and vascular features
The main analyses were performed on four different population‐based collections of primary tumours, which have been used in earlier biomarker studies (see Material and Methods and supplementary material, Table S1a–c for cohort descriptions, 5, 14). Initial analyses focused on comparing different vascular and fibroblast‐dominated stroma features between colorectal cancer (CRC), renal cell cancer (RCC) and ovarian cancer (OC; Figure 1A–D,G). Data were also used to compare intra‐case heterogeneity of vascular and perivascular features (Figure 1E,F,H; see Materials and Methods and 14 for details concerning the metrics ‘vessel size IQR’ and ‘perivascular intensity [PVI] IQR’).
Fibroblast‐rich stroma
RCC displayed the statistically significantly highest expression levels of PDGFR‐β in the tumour stroma (Figure 1A). The fraction of tumour area positive for the fibroblast marker PDGFR‐β was highest in CRC (Figure 1B). Examples of cases with significantly high and low PDGFR‐β stroma fraction, from each of the three tumour types, are shown in supplementary material, Figure S1. Independent analyses using α‐smooth muscle actin as a fibroblast marker yielded similar results when tumour types were analyzed with regard to abundance of fibroblast‐rich stroma (supplementary material, Figure S2a).
Vessels
RCC cases displayed statistically significantly higher vessel density as compared to OC and CRC (Figure 1C). Concerning vessel size, analyses showed that both absolute values and intra‐case variation of vessel size were larger in OC in comparison to CRC and RCC (Figure 1D,E).
Perivascular cells
CRC cases showed significantly higher absolute values and intra‐case variation of perivascular PDGFR‐β expression (Figure 1G,H). Examples of cases with high and low PDGFR‐β perivascular intensity, from each of the three tumour types, are shown in supplementary material, Figure S3. Independent analyses using α‐SMA as a marker for perivascular cells demonstrated that the tumour‐type‐related differences were not detected when this marker was used for perivascular cells (supplementary material, Figure S2b).
Associations between stroma and vascular metrics in each of the analyzed tumour types
Case‐based values for vessel density, vessel size, and perivascular status were compared with regard to pair‐wise correlation within the different tumour types (Figure 2A, right part). Associations between vessel densities, vessel size and perivascular status were in general low, as described in our previous publications. However, in RCC, but not in CRC and OC, cases with higher vessel density were also characterized by lower perivascular status, resulting in moderate negative correlation (Spearman r = −0.43) between these two metrics (Figure 2A, right part).
Vessel characteristics were also compared to PDGFR‐β status in tumour fibroblast‐dominated stroma (Figure 2A, left part). These metrics showed strong positive correlations (Spearman r = 0.71, 0.67 and 0.83, respectively).
For additional analyses of potential relationships between vessel size and perivascular status, data were analysed in a vessel‐based, rather than case‐based manner. As shown in Figure 2B, these analyses confirmed the previous case‐based findings of the absence of strong correlation between vessel size and perivascular status in OC and RCC. In contrast, vessels in CRC displayed a more complicated pattern: perivascular expression of PDGFR‐β is highest in vessels of 5–15 µm diameter. For vessels bigger than 15 µm the intensity of the perivascular expression of PDGFR‐β appeared inversely correlated with vessel size.
High heterogeneity in vessel‐distribution is associated with poor survival in RCC and CRC
Previous analyses of the OC and RCC cohort have demonstrated that high heterogeneity of the perivascular status is associated with poor prognosis 5, 14. In the present study, we introduced a novel metric denoted ‘vessel distance IQR’, which describes the variation in distance to the closest located vessel for each vessel in a tumour sample. This novel metric was investigated regarding its association with other clinico‐pathological characteristics and with survival. Image examples of cores from the OC, RCC, and CRC cohorts with high and low ‘vessel distance IQR’ are shown in Figure 3.
Figure 3.
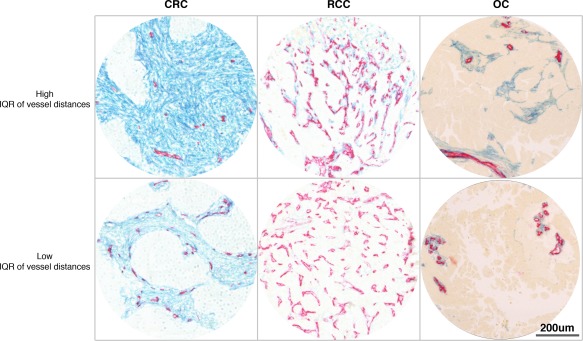
CRC, RCC, and OC cases with high or low inter‐vessel distance heterogeneity (vessel distance IQR). Photomicrographs showing examples of CRC (left), RCC (middle), and OC (right) tumours with high (upper) or low (lower) heterogeneity regarding inter‐vessel distances. Sections have been stained to detect endothelial cells with CD34 antibodies (red) and with PDGFR‐β antibodies (blue).
The associations of ‘vessel distance IQR’ with standard clinico‐pathological characteristics are shown in supplementary material, Table S2a–c. In the CRC cohort, high heterogeneity of vessel density correlated significantly with female sex, advanced T‐ and M‐ stage and low differentiation grade (supplementary material, Table S2a). In RCC, high heterogeneity of vessel density was significantly associated with male sex and high Fuhrman grade (supplementary material, Table S2b). No significant association was found in the OC cohort between ‘vessel distance IQR’ or any of the clinico‐pathological characteristics (supplementary material, Table S2c).
Notably, as shown in the Kaplan‐Meier plots of Figure 4, high heterogeneity in vessel density was significantly associated with poor survival in both RCC and CRC (median survival 95 months versus 36 month, HR = 1.95 (Confidence Intervals at 95% CI95%: 1.42–2.68), p < 0.001 and median survival 10.31 years versus 6.24 years, HR = 1.29 (CI95%: 1.00–1.67), p = 0.05, for RCC and CRC, respectively). Heterogeneity in vessel density in OC had no impact on survival (HR = 1.04 [Cl95%: 0.66–1.64], p = 0.86).
In RCC and CRC, additional analyses tested the independence of vessel distance IQR from standard clinico‐pathological characteristics, by Cox uni‐ and multi‐variate analysis. As shown in Table 1, this novel metric acted as an independent marker of prognosis in RCC (HR = 2.46 [CI95%: 1.29–4.69], p = 0.006), but not in CRC (supplementary material, Table S3).
Explorative analyses were performed to investigate if vessel density heterogeneity showed differential prognostic significance in various subsets of RCC and CRC (supplementary material, Figure S4a,b). In RCC, the strongest association between high heterogeneity of vessel density and short survival was seen in the T stage IV and in clear cell histology (supplementary material, Figure S4a). The analyses of CRC indicated that the survival association of heterogeneity of vessel density was stronger in males as compared to females (supplementary material, Figure S4b).
Discussion
Through analyses of a large dataset, obtained from state‐of‐the‐art multi‐parametric stroma‐profiling of three clinically well‐annotated tumour collections, this study identifies different stroma phenotypes in three common types of cancers.
The major findings from the comparative analyses are summarized in Table 2. The defining properties of RCC include a high vessel density together with low abundance of PDGFR‐β‐positive tumour fibroblast‐dominated stroma. Vessels of RCC are in general small and display low perivascular PDGFR‐β expression. CRC is characterized by low vessel density, high abundance of PDGFR‐β‐positive fibroblast‐dominated stroma, and high perivascular PDGFR‐β‐positivity. OC presents a third phenotype including low vessel density, larger vessel size, low perivascular PDGFR‐β expression, and moderate amounts of PDGFR‐β‐positive fibroblast‐dominated stroma. Notably, the tumour‐type‐specific differences regarding perivascular status were not detected in analyses using alpha‐smooth‐muscle actin as a perivascular marker, confirming findings that these markers detect different subsets of perivascular cells 5, 14, 21.
Table 2.
Summary of stroma features in RCC, CRC, and OC
| Metric | RCC | CRC | OC |
|---|---|---|---|
| Vessel density | High | Low | Low |
| Vessel size | Low | Low | High |
| PDGFR‐β PVI | Low | High | Low |
| Stroma abundance | Low | High | Low |
| PDGFR‐β PVI/stroma correlation | High | High | High |
| Vessel density/PDGFR‐β PVI correlation | High | Low | Low |
The tumour‐type‐specific vessel properties indicate reliance on different angiogenic programmes. The high vessel density/low perivascular coverage phenotype of RCC is compatible with VEGF‐driven angiogenesis not balanced by a vessel‐maturation programme including recruitment of PDGFR‐β‐positive perivascular cells 22, 23. RCC furthermore displays an inverse relationship between vessel density and perivascular status – a feature not observed in CRC or OC (Figure 2). This phenotype is possibly linked to the well‐established “VEGF‐high” characteristics of RCC, which occur as a consequence of VHL‐deficiency, and activation of HIF 24. In contrast, the vasculature of CRC has features of higher maturation and might thus reflect a strong involvement of physiological vascular programmes of the sub‐epithelial mucosa. This is compatible with the growth pattern of CRC, which involves invasion into the mucosa, which is composed of vascularized loose connective tissue.
Interestingly, the phenotypes emerging from these analyses correspond well to earlier described ‘fibroblast‐dominated stroma phenotypes’ which have been linked to the sensitivity to anti‐angiogenic therapy 25. The study of Smith et al, predominantly relying on analyses of different experimental tumour models, identified a ‘tumour vessel’ phenotype and ‘fibroblast‐dominated stroma vessel phenotype’ largely overlapping with the RCC‐OCand CRC phenotypes, respectively, of the present study. Notably, the ‘tumour vessel’/RCC‐OC phenotype displayed a greater sensitivity to treatment with anti‐VEGF agent, in agreement with the clinical experience of efficiency of treatment with such drugs in RCC 26 and in maintenance therapy of OC 10, 27, but not in CRC 11, 28. An implication of these findings, for possible future testing, is that ‘outliers’ of CRC with an RCC‐like tumour stroma might be sensitive to anti‐VEGF treatments. Furthermore, it could be tested whether the anti‐VEGF‐benefit in OC is particularly strong in cases displaying the most typical ‘perivascular‐low’ phenotype.
A series of recent studies have indicated that tumour metastasis relying on vessel co‐option displays a reduced sensitivity to anti‐VEGF‐agents 13, 29, 30, 31. Among the primary‐tumour‐derived vascular phenotypes of the present study, the CRC‐type vessels with high PDGFR‐β expression are most similar to the VEGF‐antagonist‐resistant co‐opted metastasis vessels. Future studies should also explore the presence of the CRC primary tumour vessel phenotype in metastatic lesions, and describe its sensitivity to VEGF‐antagonists.
Concerning mechanisms for ‘fibroblast‐dominated stroma‐building’ a strong intra‐case correlation of PDGFR‐β expression in fibroblast‐stroma and perivascular regions was noted (Figure 2, left panel). This indicates that related cells populate these compartments. This notion is supported by recent lineage‐tracing studies which have established strong links between perivascular cells and fibroblasts/myo‐fibroblasts in different settings 30, 32, 33, 34.
This study identified previously un‐recognized prognostic significance in RCC of a novel metric, ‘vessel distance IQR’, related to the distribution of vessels (Figure 4 and Table 1). It is recognized that this finding should be validated in independent RCC cohorts. It is noted as a limitation of the present study that analyses have been performed on cohorts with some samples dating back to the 1980s. Since then, new treatment strategies have been implemented such as bevacizumab and PARP‐inhibitors in OC, and tyrosine kinase inhibitors (TKIs) and check point inhibitors in RCC. Future studies on other cohorts should address potential response‐predictive significance of ‘vessel distance IQR’. Such studies, if relying mostly on analyses of primary tumours for biomarkers predicting sensitivity of metastatic lesions to drug‐of‐interest, should also integrate analyses of the ‘stability’ of vascular and fibroblast features in matched primary tumours and metastatic lesions.
The finding of prognostic significance of ‘vessel distance IQR’ illustrates the potential of digital image‐analyses‐based scoring of vascular features for the development of features or ‘metrics’ that cannot be scored or detected with conventional visual inspection of stained tumour sections. It can be envisioned that continued analyses of the existing data‐set will allow generation of additional ‘higher‐level’ features of mechanistic relevance as well as biomarker significance. Such studies should also explore to what extent variations in tumour microenvironment composition can be explained by different changes in the cancer genome.
For future studies, it is noted that the profiling of the present study can be extended to additional features, such as vascular proliferation or marker‐defined subsets of perivascular and endothelial cells. Such studies, together with the collective findings of the present work, should contribute to the long‐term goal of exploiting tumour stroma variability for clinically meaningful tumour sub‐typing and patient stratification.
Author contributions statement
SC, MF, AM: involved in study design, data collection and data analyses; GBAW, HWN, AGVdZ, KJ, BN, MJ: involved in data collection and TMA construction; PR, DE were involved in material and data collection; IH: involved data collection and data analyses; AÖ: involved in study design and data analyses. All authors contributed to writing of the manuscript. Funding was provided by research grants awarded to AÖ and HD.
Supporting information
SUPPLEMENTARY MATERIAL ONLINE
Supplementary figure legends
Figure S1. Images showing examples of cases with low (a, c and f) or high (b,d and e) PDGFR‐β stroma fraction in renal cell carcinoma (RCC) (a,b), colorectal carcinoma (CRC) (c,d) and ovarian carcinoma (OC) (e,f)
Figure S2. Box plots showing differences between the tumour types regarding a) stroma fraction and b) perivascular intensity for α‐SMA. RCC, renal cell carcinoma; OC, ovarian carcinoma
Figure S3.. Images showing examples of cases with low (a, c and f) or high (b,d and e) PDGFR‐β perivascular intensity in renal cell carcinoma (RCC) (a,b), colorectal carcinoma (CRC) (c,d) and ovarian carcinoma (OC) (e,f)
Figure S4. Associations between vessel heterogeneity of inter‐vessel‐distances and overall survival in subgroups of colorectal carcinoma and renal cell carcinoma. Forest‐plots showing the hazard ratios (HRs), including confidence intervals, for “vessel distance IQR” in subgroups of a) renal cell carcinoma and b) colorectal carcinoma
Table S1. Clinico‐pathological characteristics of patients in a) the renal cell carcinoma cohort, b) the colorectal carcinoma cohort, and c) the ovarian carcinoma cohort
Table S2. Associations with the clinico‐pathological characteristics of vessel distance IQR in a) colorectal carcinoma, b) renal cell carcinoma, and c) ovarian carcinoma
Table S3. Multivariate Cox regression analyses of high and low IQR of vessel distances in colorectal carcinoma
Acknowledgements
The study was supported by grants from The Cancer Research Foundations of Radiumhemmet, The Swedish Cancer Society, The Swedish Research Council and Stockholm City Council.
The authors acknowledge Marja Hallström for the outstanding technical support.
No conflicts of interest were declared.
References
- 1. Jain RK. Antiangiogenesis strategies revisited: from starving tumors to alleviating hypoxia. Cancer Cell 2014; 26 : 605–622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Weidner N, Semple JP, Welch WR, et al Tumor angiogenesis and metastasis–correlation in invasive breast carcinoma. N Engl J Med 1991; 324 : 1–8. [DOI] [PubMed] [Google Scholar]
- 3. Yao X, Qian CN, Zhang ZF, et al Two distinct types of blood vessels in clear cell renal cell carcinoma have contrasting prognostic implications. Clin Cancer Res 2007; 13 : 161–169. [DOI] [PubMed] [Google Scholar]
- 4. Gravdal K, Halvorsen OJ, Haukaas SA, et al Proliferation of immature tumor vessels is a novel marker of clinical progression in prostate cancer. Cancer Res 2009; 69 : 4708–4715. [DOI] [PubMed] [Google Scholar]
- 5. Corvigno S, Wisman GB, Mezheyeuski A, et al Markers of fibroblast‐rich tumor stroma and perivascular cells in serous ovarian cancer: inter‐ and intra‐patient heterogeneity and impact on survival. Oncotarget 2016; 7 : 18573–18584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Langenkamp E, Zhang L, Lugano R, et al Elevated expression of the C‐type lectin CD93 in the glioblastoma vasculature regulates cytoskeletal rearrangements that enhance vessel function and reduce host survival. Cancer Res 2015; 75 : 4504–4516. [DOI] [PubMed] [Google Scholar]
- 7. Jayson GC, Kerbel R, Ellis LM, et al Antiangiogenic therapy in oncology: current status and future directions. Lancet 2016; 388 : 518–529. [DOI] [PubMed] [Google Scholar]
- 8. Bennouna J, Sastre J, Arnold D, et al Continuation of bevacizumab after first progression in metastatic colorectal cancer (ML18147): a randomised phase 3 trial. Lancet Oncol 2013; 14 : 29–37. [DOI] [PubMed] [Google Scholar]
- 9. Stein MN, Flaherty KT. CCR drug updates: sorafenib and sunitinib in renal cell carcinoma. Clin Cancer Res 2007; 13 : 3765–3770. [DOI] [PubMed] [Google Scholar]
- 10. Burger RA, Brady MF, Bookman MA, et al Incorporation of bevacizumab in the primary treatment of ovarian cancer. N Engl J Med 2011; 365 : 2473–2483. [DOI] [PubMed] [Google Scholar]
- 11. Stathopoulos GP, Batziou C, Trafalis D, et al Treatment of colorectal cancer with and without bevacizumab: a phase III study. Oncology 2010; 78 : 376–381. [DOI] [PubMed] [Google Scholar]
- 12. Dome B, Hendrix MJ, Paku S, et al Alternative vascularization mechanisms in cancer: pathology and therapeutic implications. Am J Pathol 2007; 170: 1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Frentzas S, Simoneau E, Bridgeman VL, et al Vessel co‐option mediates resistance to anti‐angiogenic therapy in liver metastases. Nat Med 2016; 22 : 1294–1302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Frodin M, Mezheyeuski A, Corvigno S, et al Perivascular PDGFR‐beta is an independent marker for prognosis in renal cell carcinoma. Br J Cancer 2017; 116 : 195–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Mezheyeuski A, Bradic Lindh M, Guren TK, et al Survival‐associated heterogeneity of marker‐defined perivascular cells in colorectal cancer. Oncotarget 2016; 7 : 41948–41958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Larsson A, Johansson ME, Wangefjord S, et al Overexpression of podocalyxin‐like protein is an independent factor of poor prognosis in colorectal cancer. Br J Cancer 2011; 105 : 666–672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Vermeij R, de Bock GH, Leffers N, et al Tumor‐infiltrating cytotoxic t lymphocytes as independent prognostic factor in epithelial ovarian cancer with wilms tumor protein 1 overexpression. J Immunother 2011; 34 : 516–523. [DOI] [PubMed] [Google Scholar]
- 18. Glimelius B, Dahl O, Cedermark B, et al Adjuvant chemotherapy in colorectal cancer: a joint analysis of randomised trials by the nordic gastrointestinal tumour adjuvant therapy group. Acta Oncol 2005; 44 : 904–912. [DOI] [PubMed] [Google Scholar]
- 19. Paulsson J, Sjoblom T, Micke P, et al Prognostic significance of stromal platelet‐derived growth factor beta‐receptor expression in human breast cancer. Am J Pathol 2009; 175 : 334–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hubert M, Vandervieren E. An adjusted boxplot for skewed distributions. Comput Stat Data Anal 2008; 52 : 5186–5201. [Google Scholar]
- 21. Mezheyeuski A, Hrynchyk I, Karlberg M, et al Image analysis‐derived metrics of histomorphological complexity predicts prognosis and treatment response in stage II‐III colon cancer. Sci Rep 2016; 6 : 36149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Carmeliet P, Jain RK. Molecular mechanisms and clinical applications of angiogenesis. Nature 2011; 473 : 298–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kerbel RS. Tumor angiogenesis. N Engl J Med 2008; 358 : 2039–2049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Choueiri TK, Fay AP, Gagnon R, et al The role of aberrant VHL/HIF pathway elements in predicting clinical outcome to pazopanib therapy in patients with metastatic clear‐cell renal cell carcinoma. Clin Cancer Res 2013; 19 : 5218–5226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Smith NR, Baker D, Farren M, et al Tumor stromal architecture can define the intrinsic tumor response to VEGF‐targeted therapy. Clin Cancer Res 2013; 19 : 6943–6956. [DOI] [PubMed] [Google Scholar]
- 26. Escudier B, Pluzanska A, Koralewski P, et al Bevacizumab plus interferon alfa‐2a for treatment of metastatic renal cell carcinoma: a randomised, double‐blind phase III trial. Lancet 2007; 370 : 2103–2111. [DOI] [PubMed] [Google Scholar]
- 27. Perren TJ, Swart AM, Pfisterer J, et al A phase 3 trial of bevacizumab in ovarian cancer. N Engl J Med 2011; 365 : 2484–2496. [DOI] [PubMed] [Google Scholar]
- 28. Fuchs CS, Marshall J, Barrueco J. Randomized, controlled trial of irinotecan plus infusional, bolus, or oral fluoropyrimidines in first‐line treatment of metastatic colorectal cancer: updated results from the BICC‐C study. J Clin Oncol 2008; 26 : 689–690. [DOI] [PubMed] [Google Scholar]
- 29. Bridgeman VL, Vermeulen PB, Foo S, et al Vessel co‐option is common in human lung metastases and mediates resistance to anti‐angiogenic therapy in preclinical lung metastasis models. J Pathol 2017; 241 : 362–374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kramann R, Schneider RK, DiRocco DP, et al Perivascular Gli1+ progenitors are key contributors to injury‐induced organ fibrosis. Cell Stem Cell 2015; 16 : 51–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Donnem T, Hu J, Ferguson M, et al Vessel co‐option in primary human tumors and metastases: an obstacle to effective anti‐angiogenic treatment? Cancer Med 2013; 2 : 427–436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Dulauroy S, Di Carlo SE, Langa F, et al Lineage tracing and genetic ablation of ADAM12(+) perivascular cells identify a major source of profibrotic cells during acute tissue injury. Nat Med 2012; 18 : 1262–1270. [DOI] [PubMed] [Google Scholar]
- 33. Goritz C, Dias DO, Tomilin N, et al A pericyte origin of spinal cord scar tissue. Science 2011; 333 : 238–242. [DOI] [PubMed] [Google Scholar]
- 34. Humphreys BD, Lin SL, Kobayashi A, et al Fate tracing reveals the pericyte and not epithelial origin of myofibroblasts in kidney fibrosis. Am J Pathol 2010; 176 : 85–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
SUPPLEMENTARY MATERIAL ONLINE
Supplementary figure legends
Figure S1. Images showing examples of cases with low (a, c and f) or high (b,d and e) PDGFR‐β stroma fraction in renal cell carcinoma (RCC) (a,b), colorectal carcinoma (CRC) (c,d) and ovarian carcinoma (OC) (e,f)
Figure S2. Box plots showing differences between the tumour types regarding a) stroma fraction and b) perivascular intensity for α‐SMA. RCC, renal cell carcinoma; OC, ovarian carcinoma
Figure S3.. Images showing examples of cases with low (a, c and f) or high (b,d and e) PDGFR‐β perivascular intensity in renal cell carcinoma (RCC) (a,b), colorectal carcinoma (CRC) (c,d) and ovarian carcinoma (OC) (e,f)
Figure S4. Associations between vessel heterogeneity of inter‐vessel‐distances and overall survival in subgroups of colorectal carcinoma and renal cell carcinoma. Forest‐plots showing the hazard ratios (HRs), including confidence intervals, for “vessel distance IQR” in subgroups of a) renal cell carcinoma and b) colorectal carcinoma
Table S1. Clinico‐pathological characteristics of patients in a) the renal cell carcinoma cohort, b) the colorectal carcinoma cohort, and c) the ovarian carcinoma cohort
Table S2. Associations with the clinico‐pathological characteristics of vessel distance IQR in a) colorectal carcinoma, b) renal cell carcinoma, and c) ovarian carcinoma
Table S3. Multivariate Cox regression analyses of high and low IQR of vessel distances in colorectal carcinoma


