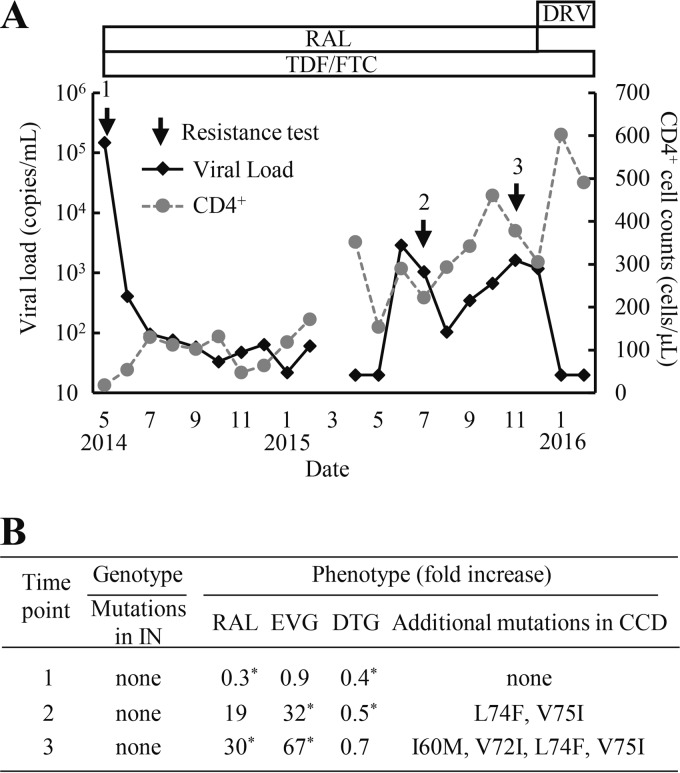
FIG 1.
Clinical course and drug resistance profiles of a patient on an RAL-based ART regimen. (A) The treatment history and clinical course. Arrows indicate the time points for drug resistance assays. (B) HIV-1 genotypic and phenotypic resistance assay results. Genotypic results were analyzed according to the major drug resistance mutation lists (HIV Drug Resistance Database at Stanford University [March 2015] and IAS-USA drug resistance mutations list [16]). Resistance levels to INSTIs were calculated as the fold increase in the EC50 of the HIV-1 variants relative to that of WT. The data shown were obtained from at least three independent experiments. Statistical significance was calculated for difference between the WT and recombinant virus derived from a clinical isolate using a Student t test with a statistical cutoff of P < 0.02 (*). Additional mutations in the catalytic core domain (CCD) were observed in recombinant clones of time points 2 and 3 compared to time point 1 (GenBank accession no. LC201871 to LC201873).