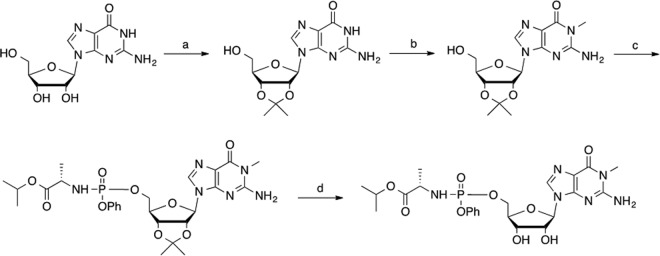
FIG 5.
Chemical synthesis of the N1-methyl-G phosphoramidate prodrug. Step a, p-toluenesulfonic acid and 2,2-dimethoxypropane in acetone, overnight at room temperature (yield, 93%); step b, NaH and MeI in dimethyl sulfoxide, overnight at room temperature (yield, 90%); step c, phosphorochloridate and N-methylimidazole in tetrahydrofuran-acetonitrile, 3 h at room temperature (yield, 91%); step d, 85% trifluoroacetic acid, 1 h at room temperature (yield, 86%).