Abstract
Background
After cancer surgery, complications and disability prevent some patients from receiving subsequent treatments. Given that an inability to complete all intended cancer therapies may negate the oncologic benefits of surgical therapy, strategies to improve Return to Intended Oncologic Treatment (RIOT), including minimally invasive surgery (MIS), are being investigated.
Methods
This project was designed to evaluate liver tumor patients to determine the RIOT rate, risk factors for inability to RIOT, and its impact on survivals. Outcomes for a homogenous cohort of 223 patients who underwent open-approach surgery for metachronous colorectal liver metastases and a group of 27 liver tumor patients treated with MIS hepatectomy were examined.
Results
Of the 223 open-approach patients, 167 were offered postoperative therapy, yielding a RIOT rate of 75%. The remaining 56 (25%) patients were unable to receive further treatment due to surgical complications (n=29 pts) or poor performance status (n= 27 pts). Risk factors associated with inability to RIOT were hypertension (OR 2.2, p=0.025), multiple preoperative chemotherapy regimens (OR 5.9, p=0.039), and postoperative complications (OR 2.0, p=0.039). Inability to RIOT correlated with shorter disease-free and overall survivals (p<.001,HR=2.16; and p=.005,HR=2.07, respectively). In contrast to the open surgery group, 100% of MIS patients who were intended to initiate postoperative therapy did so (p=0.038) within a shorter median time interval (MIS: 15 days vs. open: 42 days; p<0.001).
Conclusions
The relationship between RIOT and long-term oncologic outcomes suggests that RIOT rates for both open- and MIS-approach cancer surgery should routinely be reported as a quality indicator.
Introduction
Recent improvements in survivals for a number of malignancies can be attributed to the development of multidisciplinary treatment protocols that include surgery and systemic therapies.[1] These oncosurgical strategies frequently indicate surgical tumor resection followed by postoperative adjuvant systemic therapy. Specifically,, for patients diagnosed with liver metastases from colorectal cancer, the significant improvement in overall survival over the last two decades is attributed to the increased safety and use of hepatectomy combined with the introduction of effective drugs for systemic treatment.[2, 3] Currently, patients with resected colorectal liver metastases (CLM) demonstrate 5-year overall survivals (OS) of up to 60%.[4, 5] However, disease recurrence after liver resection remains a frequent event, with nearly two-thirds of patients developing relapse.[6] Up to 50% of recurrences occur in the liver with the majority occurring within the first 2 years after hepatectomy.[7–9]
These statistics, combined with adjuvant therapy efficacy data from patients with locally advanced colorectal cancer[10] and recently published multiinstitutional studies, support the role of adjuvant chemotherapy following resection of hepatic metastases.[3, 11] Given this paradigm, any complications and/or general disability related to the hepatectomy procedure that prevents patients from Return to Intended Oncologic Treatment (RIOT) may increase the risk of recurrence and negate some or all of the benefits of surgery. In this setting, the RIOT rate may serve as an important quality indicator for the combined oncosurgical treatment plan. As RIOT is a novel metric, factors negatively affecting RIOT and the potential impact of minimally invasive surgery (MIS) on RIOT have never been assessed.
Using a homogenous population of patients undergoing open hepatectomy for metachronous CLM as a proof-of-principle, this study was designed to identify the rate of and factors independently associated with inability to RIOT. Additionally, it aimed to evaluate the potential impact of the MIS-approach on RIOT rate and subsequent, associated, long-term oncologic outcomes.
Patients and methods
Study population
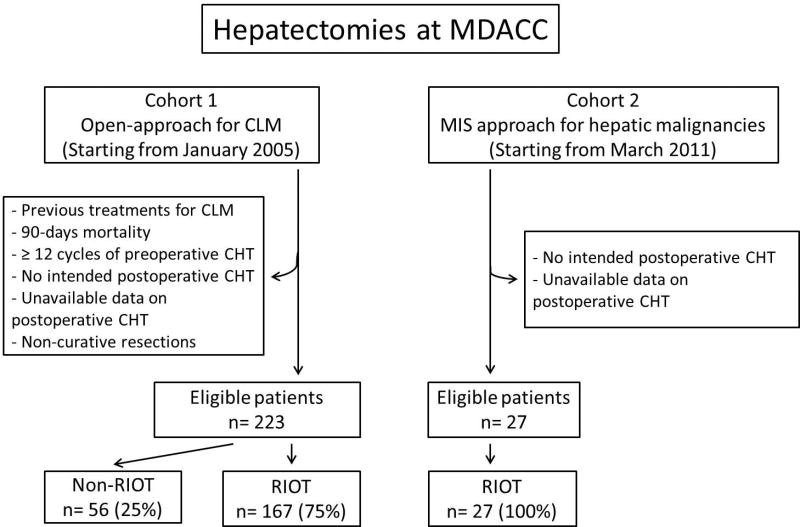
With IRB approval, the prospectively maintained liver resection database of the Department of Surgical Oncology at The University of Texas-MD Anderson Cancer Center was queried in order to identify all patients who underwent hepatectomy from January 2005 to October 2011 (n=1669). Two cohorts of patients were identified (Figure 1). The first cohort included 351 patients who underwent open-approach hepatectomy for metachronous colorectal liver metastases. After excluding patients who underwent previous liver surgery for metastatic colorectal cancer (i.e. redo resection), patients who died within 90 days after hepatectomy, patients who received more than 12 cycles of preoperative chemotherapy, patients who were not intended to receive postoperative chemotherapy, and those for whom data regarding postoperative CHT were incomplete, a homogeneous cohort of 223 patients remained (open-approach cohort). A second cohort included 27 patients who, from February 2011 to October 2012, underwent curative-intent MIS hepatectomy for various primary and metastatic malignancies (MIS cohort).
Figure 1.
Study design. Abbreviations: CLM, colorectal liver metastases; CHT, chemotherapy; RIOT, return to intended oncologic treatment.
Preoperative assessment
The pre-operative assessment included a computed tomography using liver protocol (rapid injection of 3–5 mL/sec of intravenous contrast, triple-phase imaging, and 2.5- to 5-mm slice-thickness through the liver) and/or liver protocol magnetic resonance imaging. For MR, hepatocyte-specific contrast agents were increasingly used during the study period. If the calculated standardized future liver remnant volume was inadequate, pre-operative portal vein embolization (PVE) was performed using a previously reported algorithm.[12, 13]
Surgery
In the open approach cohort, a standardized operative technique was used.[14, 15] Intraoperative hepatic ultrasonography with a 5- to 7.5-MHz probe (Aloka Co. Ltd., Tokyo, Japan) was routinely performed to confirm the preoperative imaging findings, to rule out previously undetected nodules, to visualize the relationships between the tumor and vascular and biliary structures, and to delineate the extent of hepatectomy. According to the Brisbane 2000 terminology, the resection of ≥3 segments was defined as major hepatectomy.[16] Associated procedures were defined as any intra-or extra-abdominal procedure concomitant to the hepatectomy, except hepatic artery pump placement, ablative procedure, cholecystectomy, liver wedge biopsy, ventral or umbilical hernia repair, and endoscopic evaluations.[17]
In the MIS-approach cohort, patients were placed for left liver resection in the low lithotomy position and for right hepatic resection in a left-lateral decubitus position. A 3–4 trocar approach with or without hand port placement was utilized and the intra-abdominal pressure was maintained at 12 to 15 mm Hg. Dissection of hilar, pericaval and hepatic venous structures was conducted as in open surgery. Before parenchymal dissection, an umbilical tape was passed around the hepatoduodenal ligament for inflow occlusion (Pringle maneuver), when needed. The liver parenchyma was dissected, divided and coagulated with bipolar forceps and the Ligasure device (5 mm, COVIDIEN, Boulder, CO, USA). Fibrin glue and drains were used at the surgeon’s discretion.
Description of postoperative outcomes and RIOT
Postoperative complications included postoperative adverse events resulting from the liver resection or associated procedures. Complications were classified according to a standard classification system.[18] Grade I and II complications were defined as minor complications and grade III and IV complications were defined as major complications.
Postoperative medical oncology records of all patients (open-approach and MIS-approach) included in the current study were reviewed to separate patients into two groups: those who could return to intended oncologic treatment (RIOT group) and those who could not (non-RIOT group). The exact reason(s) for failure to RIOT were documented, including postoperative complications or poor general performance status.
Statistical Analysis
Statistical analysis was performed with SPSS (version 19.0; SPSS Inc., Chicago, IL). Overall survival (OS) and recurrence-free survival (RFS) were calculated starting from the date of hepatectomy until the date of death, of radiologically or clinically confirmed recurrence, or of last follow-up, as appropriate. Continuous data were expressed as medians (range) and compared with the Mann-Whitney U test. Categorical data were compared by the Chi-squared or Fisher’s exact test as appropriate. Variables with a significant impact on the ability to RIOT in univariate analysis were entered into multivariate analysis. Multivariate analysis for predictors of the inability to RIOT and for factors associated with survivals was performed by logistic regression, with backward elimination of non-significant factors. Univariate and multivariate data are presented with (p-value, Hazard Ratio, and 95% Confidence Interval) in both text and tables. A p value < 0.05 was considered statistically significant in both univariate and multivariate analyses.
Results
Baseline characteristics of patients in the open-approach cohort and analysis of RIOT
In the open-approach cohort, the median age at the time of the hepatectomy was 62 years (range: 25–88 years) and 140 (63%) patients were male. The median BMI and ASA score were 28.4 and 3, respectively. The incidence of medical comorbidities including hypertension (51%) and diabetes (15%) was typical for this patient population. Hepatectomy was preceded by PVE in 16 (7.2%) cases and by preoperative CHT in 131 (59%) cases.
Of the 223 patients in the open approach cohort, 167 were offered postoperative therapy (156 accepted and 11 declined) yielding a RIOT rate of 75%. The inability to RIOT was observed in 56 patients (25%), and was attributed to insufficient recovery from distinct complications in 29 patients and general poor performance status in 27 patients. Analysis of potential risk factors for the inability to RIOT are summarized in Table 1. In univariate analysis, age greater than or equal to 60 years old (p<.001), hypertension (p=.005), more than one line of preoperative CHT (p<.001), and postoperative complications (p=.011) were associated with the inability to RIOT. In multivariate analysis, hypertension (p=.025, 2.16, 1.1–4.23), receiving more than one preoperative CHT regimen (p=.039, 5.92 1.1–32.21), and postoperative complications (p=.039) retained independent statistical significance for association with the inability to RIOT.
Table 1.
Clinicopathological characteristics and univariate analysis of factors associated with RIOT in the open-approach cohort.
| Inability to RIOT (n=56) |
Univariate analysis | Multivariate analysis | |||||
|---|---|---|---|---|---|---|---|
|
| |||||||
| n | % | p | OR (95% CI) | p | OR (95% CI) | ||
| Preoperative characteristics | |||||||
|
| |||||||
| Age | ≥ 60 | 42 | .005 | 2.63 (1.33–5.18) | .059 | 1.97 (0.97–4.02) | |
| < 60 | 14 | ||||||
|
| |||||||
| Sex | Male | 36 | .788 | 1.09 (0.58–2.08) | |||
| Female | 20 | ||||||
|
| |||||||
| Diabetes | Yes | 12 | .132 | 1.82 (0.83–3.9) | |||
| No | 43 | ||||||
|
| |||||||
| Hypertension | Yes | 38 | .004 | 2.52 (1.33–4.78) | .025 | 2.16 (1.1–4.23) | |
| No | 18 | ||||||
|
| |||||||
| Body mass index > 30 | Yes | 20 | .508 | 1.23 (0.66–2.31) | |||
| No | 36 | ||||||
|
| |||||||
| ASA Score >2 | Yes | 50 | .196 | 1.91 (0.71–5.32) | |||
| No | 5 | ||||||
|
| |||||||
| Preoperative CHT | Yes | 29 | .194 | 1.5 (0.81–2.76) | |||
| No | 27 | ||||||
|
| |||||||
| Multiple regimen | Yes | 6 | .006 | 9.9 (1.93 – 50) | .039 | 5.92 (1.1–32.21) | |
| No | 50 | ||||||
|
| |||||||
| Preoperative PVE | Yes | 6 | .242 | 1.8 (0.65–5.43) | |||
| No | 50 | ||||||
|
| |||||||
| Intraoperative characteristics | |||||||
|
| |||||||
| Concomitant RFA | Yes | 3 | .743 | 1.24 (0.33–4.63) | |||
| No | 53 | ||||||
|
| |||||||
| Major hepatectomy | Yes | 30 | .949 | 1.01 (0.55–1.86) | |||
| No | 26 | ||||||
|
| |||||||
| Associated procedures | Yes | 12 | .315 | 1.48 (0.68–3.17) | |||
| No | 44 | ||||||
|
| |||||||
| Operation duration ≥ 180 min | Yes | 17 | .117 | 1.69 (0.88–3.2) | |||
| No | 40 | ||||||
|
| |||||||
| IOBL ≥ 1000 ml | Yes | 3 | .177 | 3.06 (0.6–15.44) | |||
| No | 53 | ||||||
|
| |||||||
| Perioperative Transfusion | Yes | 7 | .49 | 1.31 (0.6–2.87) | |||
| No | 49 | ||||||
|
| |||||||
| Postoperative characteristics | |||||||
|
| |||||||
| Complications | Yes | 27 | .011 | 2.24 (1.21–4.17) | .039 | 2.00 (1.04–3.86) | |
| No | 29 | ||||||
|
| |||||||
| Major complications | Yes | 8 | .205 | 1.82 (0.72–4.61) | |||
| No | 48 | ||||||
Abbreviations: RIOT, return to intended oncological treatment; OR, Odd Ratio; CI, confidence interval; CHT, chemotherapy; CEA, carcinoembryonic antigen; PVE, portal vein embolization; RFA, radiofrequency ablation; IOBL, intraoperative blood loss.
Factors associated with recurrence-free and overall survival in the open-approach cohort
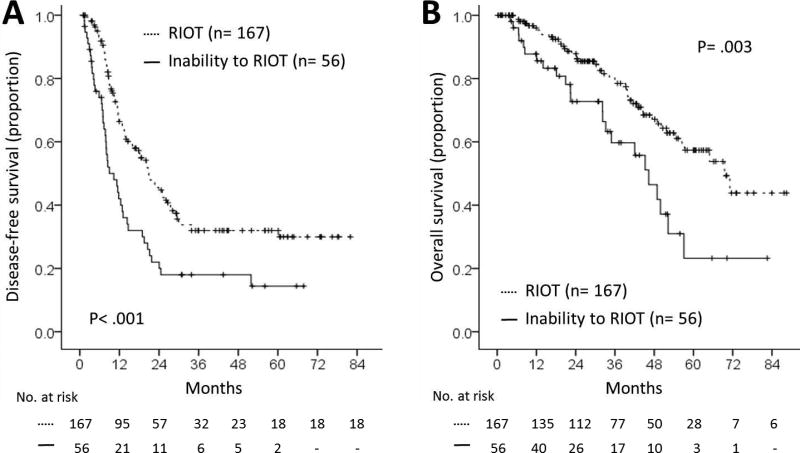
Within the open-approach cohort, those patients with the ability to RIOT had median recurrence-free and overall survivals of 22 months and 70 months, respectively. In contrast, patients who did not RIOT experienced median recurrence-free and overall survivals of only 13 months and 46 months, respectively (both p<0.01, Figure 2.)
Figure 2.
Impact of Return to Intended Oncologic Therapy (RIOT) on recurrence-free survival (A) and on overall survival (B).
Univariate analysis of risk factors potentially associated with recurrence indicated that multiple regimens of preoperative CHT (p=.001, 3.54, 1.63–7.67), multiple CLM (p=.023, 1.47, 1.05–2.06), and inability to RIOT (2.16, 2.01, 1.39–2.86) were associated with worse RFS. Mortality analysis determined that intraoperative blood loss (IOBL) >1000 ml (p= .009, 3.92, 1.41–10.85), a positive margin resection (p=.021, 2.14, 1.12–4.08), and inability to RIOT (p=.003, 2.13, 1.29–3.52) were associated with a shorter OS. When these factors were entered into multivariate analyses, multiple CLM (p=.008, 1.59, 1.13–2.23) and inability to RIOT (p=.001, 2.16, 1.48–3.13) were independently associated with a shorter RFS, while intraoperative blood loss greater than 1000 ml (p=.019, 3.44, 1.22–9.68), positive surgical margin (p=.045, 1.94, 1.02–3.74), and inability to RIOT (p=.005, 2.07 , 1.24–3.43), were independently associated with a worse OS. (Table 2, Figure 2)
Table 2.
Univariate and multivariate analyses of factors associated with recurrence-free and overall survival in the open-approach cohort.
| Variables | Univariate analysis | Multivariate analysis | ||
|---|---|---|---|---|
| p | HR (95% CI) | p | HR (95% CI) | |
| Recurrence-free survival | ||||
| ASA score > 2 | .064 | 1.66 (0.97–2.84) | .125 | - |
| Multiple CHT lines | .001 | 3.54 (1.63–7.67) | .221 | - |
| CEA > 5 | .055 | 1.38 (0.99–1.94) | .115 | - |
| PVE | .081 | 1.73 (0.93–3.22) | .659 | - |
| Largest CLM size > 5cm | .074 | 1.51 (0.96–2.36) | .515 | - |
| Multiple CLM | .023 | 1.47 (1.05–2.06) | .008 | 1.59 (1.13–2.23) |
| Inability to RIOT | <.001 | 2.01 (1.39–2.86) | <.001 | 2.16 (1.48–3.13) |
| Overall survival | ||||
| Primary tumor in rectum | .054 | 1.89 (0.99–3.61) | .064 | - |
| Largest CLM size > 5cm | .063 | 1.75 (.97–3.15) | .338 | - |
| IOBL> 1000 ml | .009 | 3.92 (1.41–10.85) | .019 | 3.44 (1.22–9.68) |
| Positive margin | .021 | 2.14 (1.12–4.08) | .045 | 1.94 (1.02–3.74) |
| Inability to RIOT | .003 | 2.13 (1.29–3.52) | .005 | 2.07 (1.24–3.43) |
Multivariate Cox regression was applied with stepwise backward selection. Factors with a p value <.01 at the univariate analysis were included in the multivariate model. Factors showing no or limited statistically significant association (p> .01) with tumor recurrence or survival were deleted from the model in a stepwise fashion. The 22 factors tested were as follows: gender, age (< vs. ≥ 60 years), ASA score (≤ vs. >2), diabetes (yes vs no), hypertension (yes vs. no), BMI (< vs. ≥ 30), primary tumor location (rectum vs. colon), primary tumor nodal status (positive vs. negative) preoperative CHT (yes vs no), multiple lines of preoperative CHT (yes vs. no), preoperative PVE (yes vs. no), CEA (≤ vs. > 5ng/dl), RFA (yes vs. no), extension of hepatectomy (major vs. minor), operation duration (≤ vs. > 180 minutes), IOBL (≤ vs > 1000 ml), largest CLM size (≤ vs. > 5 cm), number of tumors (solitary v multiple), surgical margin (negative vs. positive), perioperative transfusion (yes vs. no), any complication (yes vs. no), major complication (yes vs. no), RIOT (yes vs. no).
Abbreviations: HR, Hazard Ratio; CI: confidence interval; CHT, chemotherapy; CEA, carcinoembryonic antigen; CLM, colorectal liver metastasis; PVE, portal vein embolization; RIOT, return to intended oncological therapy; IOBL, intraoperative blood loss.
Characteristics of patients in the MIS hepatectomy cohort and comparison with the open-approach cohort
When comparing preoperative characteristics, including age, comorbidities, and prior treatments, no differences were found between open and MIS cohort patients.(Table 3) Concerning perioperative characteristics, patients in the MIS cohort were less likely to undergo major hepatectomy (7.4% vs. 54%, p<.001) compared to those in the open-approach cohort. The MIS cohort had lower median intraoperative blood loss (75 cc vs. 200 cc, p<.001) and less frequent perioperative transfusions (0% vs 17%, p=.03, respectively) (197 vs. 135 minutes, p<.001). With regard to pathologic characteristics, the rate of multifocal tumor resection was higher in the open-approach group than in the MIS cohort (39% vs. 15%, p=.014), while the median size of largest metastasis (25 mm vs. 20 mm, p=.144) and the rate of positive surgical margin (9% vs. 4%, p=.484) did not differ significantly between the two cohorts.
Table 3.
Clinicopathological characteristics of the open-approach and MIS-approach cohorts.
| Open-approach cohort | MIS-approach cohort | |||||
|---|---|---|---|---|---|---|
|
| ||||||
| No. of patients (n=223) |
% | No. of patients (n=27) |
% | p | ||
| Preoperative characteristics | ||||||
|
| ||||||
| Median age, years (range) | 62 (25 – 88) | 60 (30 – 78) | .959 | |||
|
| ||||||
| Male | Yes | 140 | 63 | 17 | 63 | .985 |
| No | 83 | 37 | 10 | 37 | ||
|
| ||||||
| Diabetes | Yes | 34 | 15 | 8 | 30 | .097 |
| No | 186 | 85 | 19 | 70 | ||
|
| ||||||
| Hypertension | Yes | 114 | 51 | 13 | 48 | .77 |
| No | 109 | 49 | 14 | 52 | ||
|
| ||||||
| Body mass index | 28.4 (17.2 – 47) | 26 (21 – 44) | .108 | |||
| Body mass index > 30 | Yes | 88 | 39 | 9 | 33 | .537 |
| No | 135 | 61 | 18 | 67 | ||
|
| ||||||
| ASA Score | 3 (2 – 4) | 3 (2 – 3) | .178 | |||
| ASA Score >2 | Yes | 191 | 86 | 25 | 93 | .551 |
| No | 32 | 14 | 2 | 7 | ||
| Preoperative CHT | Yes | 131 | 59 | 15 | 56 | .717 |
| No | 92 | 41 | 12 | 44 | ||
|
| ||||||
| Multiple regimen | Yes | 8 | 4 | 0 | 0 | .605 |
| No | 215 | 96 | 27 | 100 | ||
|
| ||||||
| Median number of cycles of preoperative CHT (Range) | 4 (0 – 10) | 1 (0 – 12) | .207 | |||
|
| ||||||
| Preoperative PVE | Yes | 16 | 7 | 0 | 0 | .230 |
| No | 207 | 93 | 27 | 100 | ||
|
| ||||||
| Intraoperative characteristics | ||||||
|
| ||||||
| Associated RFA | Yes | 14 | 6 | 0 | 0 | .375 |
| No | 209 | 94 | 27 | 100 | ||
|
| ||||||
| Liver Resection | Minor | 103 | 46 | 25 | 92.6 | <.001 |
| Major | 120 | 54 | 2 | 7.4 | ||
|
| ||||||
| Associated procedures | Yes | 38 | 17 | 4 | 15 | .770 |
| No | 185 | 83 | 23 | 85 | ||
|
| ||||||
| Median operation duration, minutes (range) | 135 (50 – 714) | 197 (85 – 593) | .01 | |||
|
| ||||||
| Median IOBL, ml (range) | 200 (0 – 1800) | 75 (0 – 350) | <.001 | |||
|
| ||||||
| Perioperative Transfusion | Yes | 37 | 17 | 0 | 0 | .03 |
| No | 186 | 83 | 27 | 100 | ||
| Pathologic characteristics | ||||||
|
| ||||||
| Median tumors number (range) | 1 (1 – 8) | 1 (1 – 4) | .01 | |||
|
| ||||||
| Median largest tumor size, mm (range) | 25 (5 – 120) | 20 (2 – 66) | .144 | |||
|
| ||||||
| Surgical margin | Positive | 21 | 9 | 1 | 4 | .484 |
|
| ||||||
| Negative | 202 | 91 | 26 | 96 | ||
|
| ||||||
| Postoperative characteristics | ||||||
|
| ||||||
| Complications | Yes | 78 | 35 | 0 | 0 | <.001 |
| No | 145 | 65 | 27 | 100 | ||
|
| ||||||
| Major complications | Yes | 22 | 10 | 0 | 0 | .144 |
| No | 201 | 90 | 27 | 100 | ||
|
| ||||||
| Median length of stay, Days (range) | 6 (3 – 46) | 4 (1 – 8) | <.001 | |||
|
| ||||||
| Ability to RIOT | Yes | 167 | 75 | 27 | 100 | .003 |
| No | 56 | 25 | 0 | 0 | ||
Values are expressed in numbers and percentages or in medians (range).
Abbreviations: MIS, minimally invasive surgery; CHT, chemotherapy; CEA, carcinoembryonic antigen; PVE, portal vein embolization; RFA, radiofrequency ablation; IOBL, intraoperative blood loss; RIOT, return to intended oncologic therapy.
Comparison of postoperative characteristics revealed that the complication rate was lower in the MIS-approach cohort compared to the open-approach cohort (0% vs. 35%, p<.001). This result paralleled a shorter median length of hospital stay (3.7 vs. 6 days, p<.001) and a higher RIOT rate in the MIS cohort (100% vs. 75%, p=.003). In addition, the median time from surgery to reinitiation of cancer therapy was significantly shorter in the MIS group (median: 15 days; range: 5–36 days), compared to the open-approach group (median: 42 days; range; 25–70 days, p<0.001).
Discussion
In this study we proposed the Return to Intended Oncologic Treatment or ‘RIOT’ as a novel metric to evaluate the quality of oncologic surgery. The results of this analysis demonstrated that one-quarter of patients undergoing open-approach hepatectomy for metachronous CLM were unable to return to intended oncologic therapies, leading to a RIOT rate of 75%. Inability to RIOT was higher in patients who underwent multiple regimens of preoperative CHT, were affected by pre-existent hypertension, and in those who had postoperative complications. Furthermore, the inability to RIOT was independently associated with both shorter RFS and OS.
Cancer survivorship after surgical oncology procedures is dependent on a mix of patient comorbidity, tumor biology, and impact of surgery on recovery. In an attempt to dissect this milieu, the secondary analysis of this study examined a cohort of patients undergoing MIS liver resection for malignancy. Clearly, this was a selected group that contained patients who required less extensive hepatectomy when compared to the open-approach cohort. This analysis determined that a significantly higher percentage of patients in the MIS group who were intended to RIOT did accomplish this milestone. Despite similar age and comorbidity profiles, the MIS group demonstrated a RIOT rate of 100% and, more importantly, a nearly 4 week reduction in time to reinitiation of cancer treatment. Accepting the differences in magnitude of liver resection, we interpret these data simply as a proof-of-concept that RIOT rates may differ between surgical approaches. In as much as RIOT rates are associated with long-term outcomes this indicator may, therefore, become an important additional metric to compare open and MIS oncosurgical approaches when the patient, staging, and tumor distribution determine that each approach would be oncologically equivalent as a method to achieve R0 resection.
The use of adjuvant chemotherapy for colorectal cancer has been studied in stage III colorectal cancer, with multiple randomized clinical trials having identified a survival benefit associated with the use of adjuvant chemotherapy for patients with colorectal cancer and regional lymph node metastases.[10] These findings have provided a theoretical rationale for the utility of adjuvant chemotherapy in the setting of resected stage IV CLM. However, only four randomized clinical trials have tried to assess the role of CHT after resection of CLM: two of them included a small number of patients and were published only in the form of an abstract.[19, 20]. The remaining two trials had a larger number of participants, but were prematurely closed because of slow accrual, thus lacking statistical power to demonstrate more than a trend toward a survival difference.[21, 22]
However, a pooled analysis including 278 patients from the latter two studies showed an independent association between adjuvant chemotherapy and improved progression-free survival and overall survival, leading the authors of this study to support the use of systemic adjuvant chemotherapy after potentially curative resection of CLM.[23] The role of adjuvant CHT after curative resection of CLM has also been examined by several large retrospective, nonrandomized studies,[24–29] the majority of which concluded that a survival benefit does exist for adjuvant postoperative systemic therapy in patients with resectable colorectal liver metastasis.
Although lacking in level 1 evidence, collectively, these data suggest that a subset of patients who do not receive adjuvant systemic therapy following potentially curative hepatic metastatectomy may be at a survival disadvantage. The findings of our study, which identified an independent association between the inability to RIOT and shorter RFS and OS supports this hypothesis. Based on these data, we believe that RIOT may be a critically important factor in long-term outcomes following oncologic surgery.
We previously demonstrated this relationship in patients with pancreatic adenocarcinoma,[30] and these data were recently reconfirmed.[31] Similar to patients with pancreatic adenocarcinoma, patients with colorectal liver metastases are assumed to have at least systemic micrometastatic disease. In this setting, if effective agents are available, an inability to deliver systemic chemotherapy would logically result in a shorter disease-free survival, and a worse overall prognosis. Given this oncologic construct, data defining a known incidence of inability to receive postoperative adjuvant therapy may support delivery of neoadjuvant systemic therapy.
The current study is the first to analyze factors independently predicting an inability to RIOT after hepatectomy for malignancy and to assess the impact of MIS approaches in this context. As expected, postoperative complications correlated with the inability to RIOT, with a two-fold lower RIOT rate in patients who experienced complications. Interestingly, undergoing multiple lines of preoperative CHT was the strongest independent predictor of inability to RIOT, increasing the risk six-fold. This result could be related to the higher rate of complications in patients treated with multiple lines of preoperative CHT, compared to patients undergoing single line or no preoperative CHT. This association between long-term chemotherapy and hepatic chemotoxicity is well recognized,[32, 33] and leads us to advocate for short-course preoperative therapy whenever possible.[34]
Although this study used a retrospective analysis of RIOT rates, the pre and perioperative data was entered into our database in real-time and the RIOT data were unambiguous and easily obtained. Patients in the open cohort were subjected to strict inclusion and exclusion criteria to define a homogenous metachronous population that would have a clear rationale for subsequent systemic therapy. In addition, we acknowledge that the number of patients in the MIS cohort was small and that the two cohorts were different in terms of extent of hepatectomy. Thus, it could be conjectured that the higher RIOT rate in the MIS cohort was related to the lower rate of major hepatectomy. However, in the open-approach cohort, major hepatectomy was not a predictor of inability to RIOT nor of postoperative complications, suggesting that MIS approaches may have an impact on RIOT rates that is independent of magnitude of hepatectomy. Lastly, the RIOT metric only addressed reinitiation of systemic therapy. Future studies, focusing on the ability to complete the intended systemic therapy regimen, a relevant metric in other malignancies,[35–38] are needed to determine the minimum chemotherapy doses required to achieve a survival benefit in patients with resected colorectal cancer liver metastases.
In summary, this study represents an initial exploration of a novel quality metric in surgical oncology. Using open-approach and MIS hepatectomy for malignancy as a proof-of-principle, we determined that the RIOT rate may have value in assessing oncosurgical treatment strategies in multiple tumor types and in the direct comparison of open and MIS approaches to cancer surgery. If MIS approaches to malignant disease can achieve equivalent resection margins and nodal recovery, a lower rate of postoperative complications, and shorter lengths of perioperative disability, a higher RIOT rate may indicate an oncologic advantage of MIS over open approaches.
Synopsis.
The ability to return to intended oncologic therapy (RIOT) after cancer surgery is associated with improved outcomes. Given the importance of this metric, RIOT rates should be reported as a quality indicator in surgical oncology.
Acknowledgments
Source of Funding: This research was supported in part by National Institutes of Health Grant, CA016672.
Footnotes
Presented at the Annual Meeting of the Society of Surgical Oncology, March 8, 2013, National Harbor, MD
References
- 1.Bowater RJ, Abdelmalik SM, Lilford RJ. Efficacy of adjuvant chemotherapy after surgery when considered over all cancer types: a synthesis of meta-analyses. Ann Surg Oncol. 2012;19:3343–3350. doi: 10.1245/s10434-012-2388-1. [DOI] [PubMed] [Google Scholar]
- 2.Kopetz S, Chang GJ, Overman MJ, et al. Improved survival in metastatic colorectal cancer is associated with adoption of hepatic resection and improved chemotherapy. J Clin Oncol. 2009;27:3677–3683. doi: 10.1200/JCO.2008.20.5278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Adam R, De Gramont A, Figueras J, et al. The oncosurgery approach to managing liver metastases from colorectal cancer: a multidisciplinary international consensus. Oncologist. 2012;17:1225–1239. doi: 10.1634/theoncologist.2012-0121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Choti MA, Sitzmann JV, Tiburi MF, et al. Trends in long-term survival following liver resection for hepatic colorectal metastases. Ann Surg. 2002;235:759–766. doi: 10.1097/00000658-200206000-00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fernandez FG, Drebin JA, Linehan DC, et al. Five-year survival after resection of hepatic metastases from colorectal cancer in patients screened by positron emission tomography with F-18 fluorodeoxyglucose (FDG-PET) Ann Surg. 2004;240:438–447. doi: 10.1097/01.sla.0000138076.72547.b1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.D'Angelica M, Kornprat P, Gonen M, et al. Effect on outcome of recurrence patterns after hepatectomy for colorectal metastases. Ann Surg Oncol. 2011;18:1096–1103. doi: 10.1245/s10434-010-1409-1. [DOI] [PubMed] [Google Scholar]
- 7.Fong Y, Cohen AM, Fortner JG, et al. Liver resection for colorectal metastases. J Clin Oncol. 1997;15:938–946. doi: 10.1200/JCO.1997.15.3.938. [DOI] [PubMed] [Google Scholar]
- 8.Petrelli NJ. Perioperative or adjuvant therapy for resectable colorectal hepatic metastases. J Clin Oncol. 2008;26:4862–4863. doi: 10.1200/JCO.2008.18.5868. [DOI] [PubMed] [Google Scholar]
- 9.Nakajima Y, Nagao M, Ko S, et al. Clinical predictors of recurrence site after hepatectomy for metastatic colorectal cancer. Hepatogastroenterology. 2001;48:1680–1684. [PubMed] [Google Scholar]
- 10.Allegra CJ, Yothers G, O'Connell MJ, et al. Phase III trial assessing bevacizumab in stages II and III carcinoma of the colon: results of NSABP protocol C-08. J Clin Oncol. 2011;29:11–16. doi: 10.1200/JCO.2010.30.0855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.de Jong MC, Pulitano C, Ribero D, et al. Rates and patterns of recurrence following curative intent surgery for colorectal liver metastasis: an international multi-institutional analysis of 1669 patients. Ann Surg. 2009;250:440–448. doi: 10.1097/SLA.0b013e3181b4539b. [DOI] [PubMed] [Google Scholar]
- 12.Madoff DC, Abdalla EK, Gupta S, et al. Transhepatic ipsilateral right portal vein embolization extended to segment IV: improving hypertrophy and resection outcomes with spherical particles and coils. J Vasc Interv Radiol. 2005;16:215–225. doi: 10.1097/01.RVI.0000147067.79223.85. [DOI] [PubMed] [Google Scholar]
- 13.Shindoh J, Truty MJ, Aloia TA, et al. Kinetic growth rate after portal vein embolization predicts posthepatectomy outcomes: toward zero liver-related mortality in patients with colorectal liver metastases and small future liver remnant. J Am Coll Surg. 2013;216:201–209. doi: 10.1016/j.jamcollsurg.2012.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chang SB, Palavecino M, Wray CJ, et al. Modified Makuuchi incision for foregut procedures. Arch Surg. 2010;145:281–284. doi: 10.1001/archsurg.2010.7. [DOI] [PubMed] [Google Scholar]
- 15.Aloia TA, Zorzi D, Abdalla EK, Vauthey JN. Two-surgeon technique for hepatic parenchymal transection of the noncirrhotic liver using saline-linked cautery and ultrasonic dissection. Ann Surg. 2005;242:172–177. doi: 10.1097/01.sla.0000171300.62318.f4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Terminology of liver anatomy and resections. HPB. 2000;2:333–339. doi: 10.1080/136518202760378489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zimmitti G, Roses RE, Andreou A, et al. Greater complexity of liver surgery is not associated with an increased incidence of liver-related complications except for bile leak: an experience with 2,628 consecutive resections. J Gastrointest Surg. 2013;17:57–64. doi: 10.1007/s11605-012-2000-9. discussion p 64-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240:205–213. doi: 10.1097/01.sla.0000133083.54934.ae. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.O'Connell MJ, Adson MA, Schutt AJ, et al. Clinical trial of adjuvant chemotherapy after surgical resection of colorectal cancer metastatic to the liver. Mayo Clin Proc. 1985;60:517–520. doi: 10.1016/s0025-6196(12)60567-9. [DOI] [PubMed] [Google Scholar]
- 20.Lopez-Ladron A. Observation versus postoperative chemotherapy after resection of liver metastases in patients with advanced colorectal cancer. Proc Amer Soc Clin Oncol. 2003;22:373. [Google Scholar]
- 21.Langer B. (FU) plus l-leucovorin (l-LV) versus observation after potentially curative resection of liver or lung metastases from colorectal cancer (CRC): Results of the ENG (EORTC/NCIC CTG/GIVIO) randomized trial. Proc Amer Soc Clin Oncol. 2002;21:149a. [Google Scholar]
- 22.Portier G, Elias D, Bouche O, et al. Multicenter randomized trial of adjuvant fluorouracil and folinic acid compared with surgery alone after resection of colorectal liver metastases: FFCD ACHBTH AURC 9002 trial. J Clin Oncol. 2006;24:4976–4982. doi: 10.1200/JCO.2006.06.8353. [DOI] [PubMed] [Google Scholar]
- 23.Mitry E, Fields AL, Bleiberg H, et al. Adjuvant chemotherapy after potentially curative resection of metastases from colorectal cancer: a pooled analysis of two randomized trials. J Clin Oncol. 2008;26:4906–4911. doi: 10.1200/JCO.2008.17.3781. [DOI] [PubMed] [Google Scholar]
- 24.Parks R, Gonen M, Kemeny N, et al. Adjuvant chemotherapy improves survival after resection of hepatic colorectal metastases: analysis of data from two continents. J Am Coll Surg. 2007;204:753–761. doi: 10.1016/j.jamcollsurg.2006.12.036. discussion 761-753. [DOI] [PubMed] [Google Scholar]
- 25.Fong Y, Fortner J, Sun RL, et al. Clinical score for predicting recurrence after hepatic resection for metastatic colorectal cancer: analysis of 1001 consecutive cases. Ann Surg. 1999;230:309–318. doi: 10.1097/00000658-199909000-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Figueras J, Torras J, Valls C, et al. Surgical resection of colorectal liver metastases in patients with expanded indications: a single-center experience with 501 patients. Diseases of the colon and rectum. 2007;50:478–488. doi: 10.1007/s10350-006-0817-6. [DOI] [PubMed] [Google Scholar]
- 27.Wang X, Hershman DL, Abrams JA, et al. Predictors of survival after hepatic resection among patients with colorectal liver metastasis. British journal of cancer. 2007;97:1606–1612. doi: 10.1038/sj.bjc.6604093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kim SY, Kim HJ, Hong YS, et al. Resected colorectal liver metastases: does the survival differ according to postoperative chemotherapy regimen? Journal of surgical oncology. 2009;100:713–718. doi: 10.1002/jso.21403. [DOI] [PubMed] [Google Scholar]
- 29.Ychou M, Hohenberger W, Thezenas S, et al. A randomized phase III study comparing adjuvant 5-fluorouracil/folinic acid with FOLFIRI in patients following complete resection of liver metastases from colorectal cancer. Ann Oncol. 2009;20:1964–1970. doi: 10.1093/annonc/mdp236. [DOI] [PubMed] [Google Scholar]
- 30.Aloia TA, Lee JE, Vauthey JN, et al. Delayed recovery after pancreaticoduodenectomy: a major factor impairing the delivery of adjuvant therapy? J Am Coll Surg. 2007;204:347–355. doi: 10.1016/j.jamcollsurg.2006.12.011. [DOI] [PubMed] [Google Scholar]
- 31.Merkow RP, Bilimoria KY, Tomlinson JS, et al. Postoperative Complications Reduce Adjuvant Chemotherapy Use in Resectable Pancreatic Cancer. Ann Surg. 2013 doi: 10.1097/SLA.0000000000000378. [DOI] [PubMed] [Google Scholar]
- 32.Aloia T, Sebagh M, Plasse M, et al. Liver histology and surgical outcomes after preoperative chemotherapy with fluorouracil plus oxaliplatin in colorectal cancer liver metastases. J Clin Oncol. 2006;24:4983–4990. doi: 10.1200/JCO.2006.05.8156. [DOI] [PubMed] [Google Scholar]
- 33.Vauthey JN, Pawlik TM, Ribero D, et al. Chemotherapy regimen predicts steatohepatitis and an increase in 90-day mortality after surgery for hepatic colorectal metastases. J Clin Oncol. 2006;24:2065–2072. doi: 10.1200/JCO.2005.05.3074. [DOI] [PubMed] [Google Scholar]
- 34.Kopetz S, Vauthey JN. Perioperative chemotherapy for resectable hepatic metastases. Lancet. 2008;371:963–965. doi: 10.1016/S0140-6736(08)60429-8. [DOI] [PubMed] [Google Scholar]
- 35.Bonadonna G, Moliterni A, Zambetti M, et al. 30 years' follow up of randomised studies of adjuvant CMF in operable breast cancer: cohort study. BMJ. 2005;330:217. doi: 10.1136/bmj.38314.622095.8F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bonadonna G, Valagussa P. Dose-response effect of adjuvant chemotherapy in breast cancer. The New England journal of medicine. 1981;304:10–15. doi: 10.1056/NEJM198101013040103. [DOI] [PubMed] [Google Scholar]
- 37.Weycker D, Barron R, Edelsberg J, et al. Incidence of reduced chemotherapy relative dose intensity among women with early stage breast cancer in US clinical practice. Breast Cancer Res Treat. 2012;133:301–310. doi: 10.1007/s10549-011-1949-5. [DOI] [PubMed] [Google Scholar]
- 38.Kwak LW, Halpern J, Olshen RA, Horning SJ. Prognostic significance of actual dose intensity in diffuse large-cell lymphoma: results of a tree-structured survival analysis. J Clin Oncol. 1990;8:963–977. doi: 10.1200/JCO.1990.8.6.963. [DOI] [PubMed] [Google Scholar]