Abstract
Brain tumors, which are typically very heterogeneous at the cellular level, appear to have a stem cell foundation. Recently, investigations from multiple groups have found that human as well as experimental mouse brain tumors contain subpopulations of cells that functionally behave as tumor stem cells, driving tumor growth and generating tumor cell progeny that form the tumor bulk, but which then lose tumorigenic ability. In human glioblastomas, these tumor stem cells express neural precursor markers and are capable of differentiating into tumor cells that express more mature neural lineage markers. In addition, modeling brain tumors in mice suggests that neural precursor cells more readily give rise to full blown tumors, narrowing potential cells of origin to those rarer brain cells that have a proliferative potential. Applying stem cell concepts and methodologies is giving fresh insight into brain tumor biology, cell of origin and mechanisms of growth, and is offering new opportunities for development of more effective treatments. The field of brain tumor stem cells remains very young and there is much to be learned before these new insights are translated into new patient treatments.
Keywords: Brain tumor, Stem cell, Cancer stem cell
1. Introduction
Brain tumors are aggressive neoplasms afflicting both children and adults. The adult glioblastoma, despite recent advances in chemotherapy, still has a very poor outcome with only a median survival of 15 months (Paulino and Teh, 2005; Stupp et al., 2005). As a heterogeneous group of tumors comprised of different phenotypes, brain cancers are a leading cause of cancer death in children, and one of the commonest causes of cancer death under the age 40. Children who survive their brain cancers (mainly medulloblastomas) often suffer substantial adverse effects related to the toxicities of therapy on the developing nervous system.
Malignant brain tumors, particularly glioblastomas, are extraordinarily difficult to treat for many reasons (Wen and Kesari, 2008). They arise in critical functional areas of the brain that offer formidable technical challenges for surgical resection, and they have a propensity to be infiltrate beyond the visible margins demonstrated on MRI imaging. There are few known risk factors, no preventive strategies, and no practical method for screening. There is rarely an opportunity to study the tumor tissue of early lesions, nor is there easy access to tumor tissues at different stages of treatment to assess biological responses to therapy. For adult low grade fibrillary gliomas, which typically become malignant after many years, there remains controversy whether even intervening at an early stage leads to a better patient outcome. Brain tumors are, along with pancreatic cancer, one of the most difficult human cancer problems.
In the past few years, however, due to the application of the conceptual and methodologic framework of stem cell biology (Dirks, 2008a; Pardal et al., 2003; Reya et al., 2001; Visvader and Lindeman, 2008; Ward and Dirks, 2006; Zhou et al., 2009), together with the emergence of genome wide data(2008; Huse and Holland, 2010; Parsons et al., 2008), it appears we may be getting a better understanding of the cellular and molecular mechanisms involved in brain tumor initiation and growth. These studies offer more hope that we will be able to develop more effective therapies, but at this point huge challenges remain in our understanding to translate new insight into better treatment and patient outcomes.
2. Brain tumors as stem cell problems
Human brain tumors, on the heels of work in human breast cancer (Al‐Hajj et al., 2003), were among the first solid tumors in which a cellular hierarchy for tumor initiation, utilizing prospective cell sorting and limit dilution analysis in vivo, was demonstrated (Singh et al., 2004). These brain tumor subpopulations, enriched for stem cell activity, have been shown to be resistant to conventional treatments (Bao et al., 2006a) and may maintain localization within a vascular niche (Bao et al., 2006b; Calabrese et al., 2007; Gilbertson and Rich, 2007), which is reminiscent of the normal neural stem cell niche (Shen et al., 2004, 2008, 2008). Genetically engineered mouse models of brain tumors have been shown to also maintain a hierarchy for tumor initiation (Alcantara Llaguno et al., 2009; Read et al., 2009; Ward et al., 2009), and these models strongly point to normal brain precursors, either stem cells or progenitor cells, as cells of origin (Alcantara Llaguno et al., 2009; Yang et al., 2008). Improved methodologies for culturing brain tumor precursors, adapted from normal neural stem cell biology, either in adherent or sphere based conditions in chemically defined media, are offering opportunities for chemical and genetic screening for drug discovery and new molecular target identification (Diamandis et al., 2007, 2009, 2009, 2009). Serum derived cell lines have been shown to be poorly representative of the patient brain tumor, from both a genotypic and phenotypic perspective (Lee et al., 2006). These research findings and the continued adaptation of new experimental methods have changed the field of brain tumor research. Although there remains controversy about details about the precise identity of brain tumor initiating cells, particularly with respect to cell surface markers, there is no doubt that brain tumor research has benefited by adopting a stem cell perspective.
One important point to reflect on, in trying to reconcile recent genome wide findings about brain tumors with recent stem cell insight, is that the key glioblastoma identified cancer pathways are in fact stem cell pathways. You cannot separate them completely. For the main tumor suppressor pathways implicated in brain tumorigenesis, p53, PTEN, and pRB‐p16; all pathways are involved in control of normal neural precursors, and loss of function in mice is associated with a proliferative expansion of the neural precursor compartment in the brain (Gil‐Perotin et al., 2006, 2006, 2001, 2006, 2006). These data suggest that the development of brain tumors may first require an expansion of the neural precursor compartment before full blown neoplastic transformation occurs.
Given the amount of progress in research on brain tumor stem cells, in part because of the flexibility of assay systems in vitro and in vivo, there is a great deal of attention being focused on the brain tumor field to inform the relatively new field of cancer stem cell biology, at least in solid tumors.
3. Brain tumor hierarchy and enrichment of tumor initiating cells
An idea that brain precursor cells are connected to brain cancer is not new, based on longstanding pathological observations of brain tumors (I strongly recommend reading the now out of print neuropathology textbook of Russell and Rubenstein) (Russell and Rubenstein, 1989). Experimental mouse models of brain tumors have long pointed to brain precursor zones as sites of origin of brain tumors in response to viruses or chemical carcinogenesis (reviewed previously in detail (Dirks, 2008b)). However, the discovery of precursor cells in the postnatal mammalian brain, coupled with the development of techniques to prospectively isolate these cells using magnetic bead or fluorescence activated cell sorting (FACS), and study them in appropriate stem cell assays in vitro and in vivo (Figure 1), has led to a prominent emergence and reporting of stem cell studies of human brain tumors and experimental brain tumors generated in mice.
Figure 1.
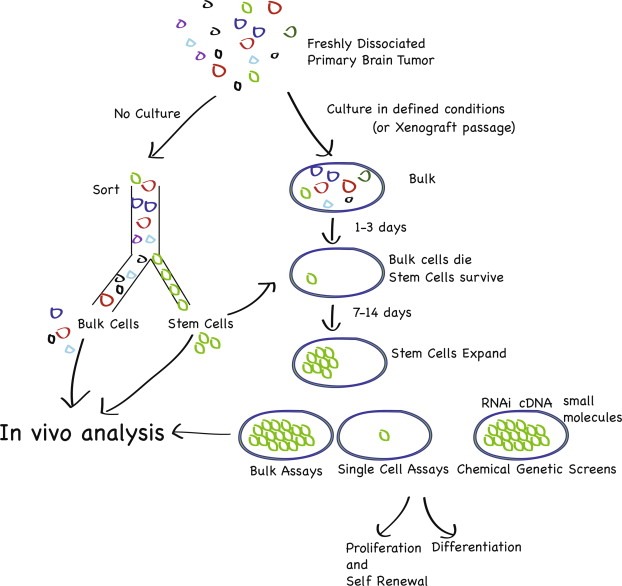
Brain tumor stem cell assay development. Brain tumor stem cells can be interrogated in stem cell assays in vivo and in vitro. The gold standard for identification of a cancer stem cell involves a sort of the stem cell population from the bulk population directly from freshly isolated tissue, and then analysis compared to bulk in an in vivo orthotopic transplantation assay. Cancer stem cells can also be isolated by selection in culture, in defined media with growth factors in the absence of serum. Fresh tumors can also be xenografted directly to expand tumor cell populations, but this method may also select for populations favored to survive in immunodeficient mice. Therefore, only a fresh sort allows comparison between putative stem cell population and bulk population. A full hierarchy of the original patient tumor is no longer available after culture, and possibly, after xenografting. Stem cells in vitro, however, give opportunities to probe mechanisms of self renewal, proliferation and differentiation, as well as to perform chemical and genetic screens. Findings on in vitro systems must be validated in vivo, ideally back to freshly sorted cells.
A few years ago, several groups attempted to grow human brain tumor cells in serum free media containing EGF and FGF, along the lines initially demonstrated by Reynolds and Weiss (Reynolds and Weiss, 1992). These groups virtually simultaneously demonstrated an ability of these cells to grow as replate‐able neurospheres, with cells expressing neural precursor markers such as nestin, and also demonstrating a capacity to differentiate in vitro (Galli et al., 2004; Hemmati et al., 2003; Ignatova et al., 2002; Singh et al., 2003). As only a limited number of the tumor cells are capable of proliferating in these conditions, as demonstrated by in vivo limit dilution analysis (Singh et al., 2003), it is clear that culture represents a strong selection strategy favoring the growth and survival of subpopulations of tumor cells, that have a precursor phenotype, that respond to the culture conditions. Therefore, the vast majority of the original patient tumor cells are not maintained in a mitogen supplemented serum free culture, as these bulk cells from the patient tumor do not proliferate in culture. The full tumor hierarchy is therefore not accessible in a culture situation.
Although this is the most likely interpretation of the effects of culture, it remains possible that culture may enable growth of tumors cells that are not capable of growing in the patient, as EGF/FGF may promote a “dedifferentiation” of populations (see (Conti and Cattaneo, 2010) for a discussion of neural stem cell culture systems and their caveats), also distorting the hierarchy in the culture system from that which exists in the patient. As well, on the other side of the coin, another possibility remains that a tumor subpopulation that is not capable of being read out in a cell culture assay still has capacity to initiate tumor formation in the patient themselves, or in an experimental in vivo assay. Caution is therefore recommended when interpreting tumor hierarchy or stem cell properties solely in culture, and extrapolation of findings in a culture to the primary patient tumor has important limitations. However, the development of defined culture conditions has been critically important for the emergence of further insight into neural stem cell and brain tumors stem cell biology, as cells with a precursor phenotype are maintained more or less (Lee et al., 2006; Pollard et al., 2009b). Glioblastoma stem cells may be better grown more efficiently in EGF/FGF in adherent conditions on laminin, facilitating chemical and genetic screens (Pollard et al., 2006, 2009, 2010). However, a prospective method for identifying the stem cell, where the cells do not see culture, remains essential to probe its functional properties.
4. Prospective sorting for brain tumor initiating cells
Prospective sorting of uncultured human brain tumor cells was first demonstrated by sorting for the CD133 antigen (Singh et al., 2004), which had been previously shown to enrich for neurosphere initiating cells from the human fetal brain (Uchida et al., 2000), as well as being a marker for human hematopoietic stem cells (Miraglia et al., 1997; Yin et al., 1997). Prominin1 (the name of the CD133 protein) had also been shown to mark cells lining the ventricle in mice (Weigmann et al., 1997), but importantly, reagents developed for human and mouse cells are very different, with antibodies against human identifying a glycosylated epitope while those against mouse Prominin1 do not. CD133+ fresh uncultured medulloblastoma and glioblastoma cells could initiate tumors after injection of small numbers of cells into the brains of NOD/SCID mice, but not CD133− cells, even though viable human cells could be found in the brain months after CD133− cell injections (Singh et al., 2004). These CD133+ cells also had an ability to initiate primary tumor sphere formation as well, but CD133− cells could not, suggesting, that these populations were reading out similar properties, clonogenicity in vitro as well as tumorigenicity in vivo (Singh et al., 2003). In another important demonstration of stem cell properties, Vescovi's group showed that single cells plated out from established glioblastoma spheres could initiate new spheres that then were tumorigenic in vivo. This experiment was important as ultimately the tumors were shown to have derived from single cells (Galli et al., 2004), and single cell tumor initiation is the sought after standard for a cancer stem cell functional analysis. Several studies have now suggested that the fraction of CD133+ cells in a human brain tumor is correlated with patient outcome (Beier et al., 2008b; Pallini et al., 2008; Rebetz et al., 2008; Thon et al., 2010; Zeppernick et al., 2008). An ability to grow as spheres in EGF/FGF media, may also identify patients who follow a more aggressive clinical course (Laks et al., 2009).
Following these demonstrations that CD133 is a marker capable of enriching for brain tumor initiating cells, a number of reports have suggested that for some brain tumors, tumor initiating ability resides in both CD133+ and CD133− fractions (Beier et al., 2007; Chen et al., 2010; Clement et al., 2009; Joo et al., 2008; Wang et al., 2008a). These data suggest that other markers must be sought. In a study by Beier et al. (2007), both CD133+ and CD133− cells derived from tumor sphere cultures were shown to be tumorigenic, however, a comparison was not made on fresh uncultured sorted populations. An important finding in this study is that portion of patient glioma cells do not express CD133 preculture and they generate entirely negative CD133− cultures, that can engraft immunodeficient mice, strongly suggesting that for at least some malignant gliomas, other markers need to be sought. Interestingly, in patient samples, Beier's group suggests that CD133 expression correlates with patient prognosis for malignant oligodendrogliomas, another suggestion that markers may be different in vivo compared to in vitro (Beier et al., 2008b).
A very recent report from the Phillips group suggested that both CD133+ and CD133− cells from multiple patient glioblastoma tumors could form neurospheres in culture, but rigorous quantitative primary in vitro limit dilution analyses were not presented, and direct comparison of uncultured sorted cells in an in vivo assay were not shown (Chen et al., 2010). In this study, sphere based cultured lines contained tumorigenic CD133+ and CD133− populations, that demonstrated different phenotypic behaviors. In some tumors that we have studied, we also see CD133+ cells as a vanishingly rare fraction, but tumor initiating activity exists in cells that are CD133− (Pollard et al., 2009b). Therefore, what is clear from these several studies is that CD133 does not define a tumor initiating population from all patient samples of glioma. As well, it is also apparent that in samples that do have a CD133+ fraction in the uncultured patient sample, once serum free cell cultures are established, CD133 may no longer identify a unique tumor subpopulation, as both CD133+ and CD133− cultured cells show tumorigenic activity. However, as discussed above, the significance of these findings based on cell populations that have been selected in culture is uncertain. Additional studies suggesting that the two fractions with respect to CD133 do not distinguish tumorigenicity have also been reported, with some data on fresh sorted samples (Clement et al., 2009; Joo et al., 2008; Wang et al., 2008a). One must, however, maintain caution regarding an evaluation of the usefulness of CD133 or any other cell surface marker in tumor cells that have been cultured, even for relatively brief periods. In culture conditions, it is also our experience that markers lose their ability to define populations with different clonogenic or tumorigenic abilities. In adherent culture, CD133− populations of normal human neural stem cells are also clonogenic and tripotent (Sun et al., 2009).
In human brain tumors where CD133 is not informative in defining tumorigenic subpopulations, it is conceivable that other (yet unidentified) markers may identify a hierarchy. A fact of primary patient tumors is that clonogenic fractions are relatively rare, as defined by in vitro LDAs. In vivo LDAs with unsorted uncultured human tumors have not been published, although data support the presence of rare tumorigenic fractions in mouse tumors (ie. many cells need to be injected orthotopically to observe tumor formation) (Ward et al., 2009). The challenge remains to identify markers, expressed on fresh tumor cells, straight out of the patient, that define a tumorigenic population in vivo. Importantly, as mentioned, markers can be very different between mouse and human. Therefore, a marker that is informative in human should not necessarily be expected to be informative in the mouse, such as CD133/Prominin1. In our own experience, Prominin1 is not helpful in identifying functionally distinct tumor populations in mouse medulloblastomas, which is also reported by others (Read et al., 2009; Ward et al., 2009).
More recently, work from Fine's group suggests that the normal mouse neural stem cell marker SSEA‐1/CD15 (Capela and Temple, 2002, 2006) may be an informative marker to define tumor initiating ability in human glioblastoma, particularly in samples that do not have an identifiable CD133 population (Son et al., 2009). This finding is particularly interesting as also several groups, including our own, have shown that this marker also enriches for tumor initiating activity in cells from freshly isolated mouse medulloblastomas arising in the Ptc+/− mice (Read et al., 2009; Ward et al., 2009). In the Fine study, CD15 was expressed in every human glioblastoma studied by FACS analysis, whereas CD133 was not found to be expressed in about half of primary uncultured tumors. However, in 3/12 primary tumors, CD15 represented >50% of the cells in the tumor, suggesting that in these tumors CD15 would not be an informative marker. In two of these tumors with high CD15 fractions, CD133 represented10.4% and 6.6% of tumor cells, suggesting that CD133 might be informative in these tumors, although it was not tested. CD15 was shown to identify cells that are relatively more clonogenic than CD15− cells in culture and CD15+ cells were more tumorigenic than CD15− cells from one primary patient sample and three cultured samples. The data supports that CD15 sorting enables enrichment, but not purification of the tumor initiating population. CD133 or CD15, therefore clearly stand to be improved upon as markers of tumorigenic brain tumor cells, and remain the most useful markers identified to date. Further studies in an increasing number of human samples, also suggests some patient tumor to patient tumor variability in expression of cell surface markers (unpublished observations).
A very recent study from the Rich lab also suggests that α6 integrin may enrich for tumor initiating activity in human glioblastoma, linking the localization of glioblastoma stem cells to the perivascular niche(Calabrese et al., 2007) and tumor initiating activity (Lathia et al., 2010). α6 integrin is a component of the laminin receptor (α6β1), and laminin substrate has recently been found to be a key ingredient for the culture of relatively pure populations of normal neural stem cells and glioblastoma stem cells in adherent culture (Conti et al., 2005; Fael Al‐Mayhani et al., 2009; Hall et al., 2008; Pollard et al., 2009b). α6β1 integrin has been previously shown to expressed in the SVZ, to enrich for neural stem cell activity in vitro, and to form important interactions for the normal neural stem cell niche (Campos et al., 2004; Lathia et al., 2007; Leone et al., 2005; Loulier et al., 2009). α6 integrin is expressed in CD133+ GBM cells but seems to be depleted in CD133− cells. Sorting for α6 integrin may offer improvement over CD133 in some GBM samples that were expanded by xenograft passaging. In this study, it remains to be determined how informative this marker is in a larger sample of primary patient samples. However, this study offers therapeutic potential, as ex vivo treatment of at least some GBM spheres with α6 blocking antibody attenuates tumorigenicity in vivo.
Clearly more effort is required to define the characteristics of tumor initiating cells based on cell surface markers or functional properties. For neural stem cells, aldehyde dehydrogenase activity may be informative (Corti et al., 2006), but has not yet been widely adapted to human brain tumors (Bar et al., 2007). A2B5, a ganglioside cell surface epitope expressed on neural precursors has also been suggested to identify tumor initiating cells from human glioblastoma (Ogden et al., 2008; Tchoghandjian et al., 2010). Autofluorescence emission at 520 nm after excitation at 488 nm has also been proposed to identify subpopulations of human gliomas with tumor initiating activity (Clement et al., 2010). Several groups have identified a side population of glioma cells in mouse models that defines a tumorigenic subpopulation (Bleau et al., 2009; Harris et al., 2008). Some of the differences observed in marker expression or enrichment ability may be in part laboratory dependent, and techniques and expertise are evolving, but there are proposals to standardize the reporting of analytical flow cytometry and cell sorting data so that methods can be compared more easily between different groups (Alexander et al., 2009). Importantly, one must remain careful about extending conclusions derived from mouse to human, but findings that mouse brain tumors from several experimental models contain subpopulations of cells that have tumor initiating activity also lend support to a hierarchical model of cancer for tumor initiating ability (Bleau et al., 2009; Harris et al., 2008; Read et al., 2009; Tamase et al., 2009; Ward et al., 2009).
5. Molecular pathways and therapeutic resistance
A key premise put forward by the cancer stem cell model is that the tumor initiating population is resistant to conventional therapies. There is increasing evidence that radiation and chemotherapy treatments of brain tumors cause an increase in the relative fractions of cells that show stem cell phenotype, indicating their resistance to the treatment. The Rich lab first showed that this was true for human glioblastomas treated with radiation therapy, based on CD133 sorting (Bao et al., 2006a), and the Holland lab suggests that an increase in side population cells occurs in mouse brain tumors that are treated with chemotherapy (Bleau et al., 2009). Human glioma stem cells may activate DNA repair mechanisms more robustly than tumor bulk, and can be sensitized to radiation by treatment with checkpoint kinase inhibitors (Bao et al., 2006a).
6. Therapeutic opportunities
The cancer stem cell model suggests additional strategies for treating brain tumors based on a stem cell hierarchy (Figure 2). The presence of distinct compartments in a tumor may demand distinct treatments, thereby requiring treatments for both cancer stem cells and bulk tumor cells. A goal for brain tumor treatment must continue to be elimination of all tumor cells, as there is no evidence so far that targeting a cancer stem cell population alone can lead to a durable cancer cure. It remains unknown in human solid tumors whether there is a smaller population of cells within a putative stem cell compartment that are quiescent, analogous to the proliferative state of normal stem cell populations in normal tissues. Thus far, the evidence from the studies thus far is that the brain tumor initiating cells are proliferating. A more proliferative state of the brain tumor stem cell may offer a therapeutic window to spare normal neural stem cells. Further study is also required to understand why brain tumor stem cells do not differentiate properly. Blocked differentiation is a key hallmark of malignant tumor cells and restoration of differentiation control may offer another strategy for cancer treatment. In mice, combined (but not singular) deletion of p53 and PTEN blocks neural stem cell differentiation, as well as enhancing proliferation, through upregulation of c‐myc, which is critical effecter for the neoplastic phenotype (Zheng et al., 2008b).
Figure 2.
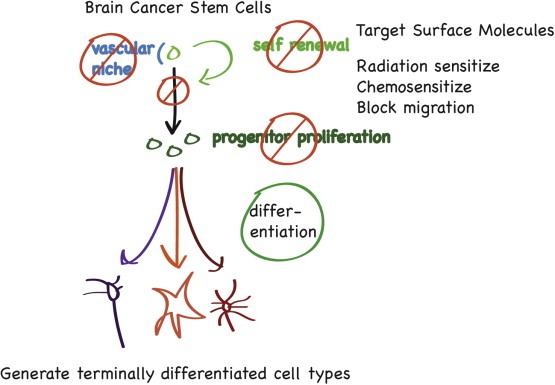
Cancer Stem Cells and Therapeutic Opportunities. From a conceptual standpoint, applying stem cell thinking to cancer opens up new therapeutic opportunities. Self renewal, as a subset of proliferation, becomes a critical process for targeting. The stem cell's supportive niche can be attacked. If one can block stem cell generation of progeny that cause clonal expansion, perhaps tumor bulk progression can be slowed. Proliferation of tumor “progenitors”, if they are more rapidly proliferative than stem cells (unproven as of yet), will be an important target. Promotion of differentiation, particularly if terminally differentiated cell types can be generated, such as neurons, may be another useful strategy. Of course, killing all tumor cells, and particularly tumor stem cells should be a goal, by further sensitizing them to conventional therapy, or targeting molecular pathways responsible for stem cell behavior. Migration, a property of normal brain precursor cells, will be important to target, if this is also a property of the cancer stem cell.
The most important therapeutic advance in glioblastoma treatment recently has been the use of upfront temozolomide chemotherapy (Stupp et al., 2005). Obviously, the modest improvement in survival of GBM patients treated with temozolomide suggests that it does satisfactorily target neither GBM stem cells, nor tumor bulk. However, data from Beier et al. (2008a) shows some human GBM stem cell activity of the drug, although other studies indicate strong resistance in a CD133+ GBM subpopulation (Liu et al., 2006). Holland's group, in a study of experimental gliomas driven by activated Akt, suggests that side population cells increase in gliomas post temozolomide treatment, further evidence that brain tumor stem cells demonstrate chemoresistance to this drug. Further studies are required to determine if the modest clinical improvements in patients treated with temozolomide reflect cancer stem cell specific activity.
Developmental signaling pathways are under intense study as pathways that can be exploited to block brain tumor stem cell self renewal and promote differentiation. Several studies now show that promotion of bone morphogenic protein (BMP) signaling can enhance brain tumor initiating cell differentiation and attenuate tumorigenic phenotype in vitro as well as in vivo (Lee et al., 2008; Piccirillo et al., 2006). Transforming growth factor‐beta (TGF‐b) and leukemia inhibiting factor (LIF) are also expressed in human glioma samples and their signaling has been shown to drive glioblastoma sphere proliferation and tumorigenicity in vitro and in vivo, and blocking either pathway attenuates tumorigenicity (Ikushima et al., 2009; Penuelas et al., 2009). Inhibition of Notch signaling, implicated in normal neural stem cell self renewal also attenuates brain tumor sphere tumorigenicity (Fan et al., 2009, 2006). Notch signaling may also play a role in radiation resistance of glioblastoma tumor initiating cells (Wang et al., 2009a). Hedgehog blockade may target tumor initiating cells in mouse medulloblastoma and in human glioma (Clement et al., 2007; Ward et al., 2009). The Rich group has also identified a number of pathways that seem to be more activated in CD133+ glioma cells, such as HIF2α (Li et al., 2009), c‐myc (Wang et al., 2008b), L1CAM (Bao et al., 2008), and Akt (Eyler et al., 2008), identifying these pathways as potential therapeutic targets. Maternal embryonic leucine zipper kinase (MELK) may also be a key regulator of brain tumor stem cell activity (Nakano et al., 2008). An additional strategy, blocking a putative niche for brain tumor stem cells, by targeting interactions with the vasculature, may also be important (Bao et al., 2006b; Borovski et al., 2009; Calabrese et al., 2007; Gilbertson and Rich, 2007; Lathia et al., 2010). In clinical trials bevacizumab (VEGF antibody) has shown some promise for GBM patients, although it may just be modifying patterns of recurrence to a more infiltrative type (Deangelis, 2010; Iwamoto et al., 2009). Finally, increasingly sophisticated small molecule or RNAi screens in neural stem cells or in glioblastoma precursors, based on advanced cultures and assays in vitro may also identify new unexpected pathways involved in stem cell proliferation and differentiation, with potential application to brain tumor treatment (Diamandis et al., 2009, 2007, 2009, 2007, 2010).
7. Brain cancer's origins: neural stem cells?
The question of brain tumor cell of origin has been a focus of intense study particularly over the past ten years. The finding of proliferative activity in cells with multilineage differentiation potential in the postnatal brain has been revolutionary. This discovery has opened the door to considering neural stem cells or their immediate downstream more proliferative progenitors as cells of origin. Although it may be quite inconceivable that a terminally differentiated projection neuron could give rise to a brain tumor, the fact that differentiated astrocytes or oligodendrocyte lineage restricted progenitors retain proliferative potential in response to injury, suggests that it is premature to ignore these populations as potential cells of origin for brain tumors. It is conceivable that tumors of different histologic types have different cells of origin, and there is also data to suggest that tumors of the same histologic type can have two different cells of origin. Identification of the cell of origin may ultimately permit earlier detection, more accurate prediction of tumor clinical behavior, or guide therapy as activation of the same oncogenic pathway may have different effects on tumor cell growth depending on cell of origin (Perez‐Losada and Balmain, 2003).
In the normal postnatal mammalian brain, stem cells are thought to reside adjacent to the cerebral ventricle, in the subventricular zone. Several studies suggest similar locations for normal human neural stem cells (Curtis et al., 2007; Sanai et al., 2004). A recent clinical study examined the location of human gliomas by MRI imaging, and found a close association in the majority of tumors to at least a point of contact to the ventricular wall, suggesting a possible origin of these tumors from the normal stem cell compartment (Barami et al., 2009). However, it is clear that other tumors have no detectable connection to the ventricle. I believe that we cannot necessarily infer cell of origin in human tumors based on the expression of lineage markers in the tumor in situ, nor the phenotype of the brain tumor stem cells or their differentiation profile. Non‐stem cells may reacquire self renewal ability or less restricted differentiation profile as part of the neoplastic transformation process, as has been shown in experimental leukemias (Krivtsov et al., 2006).
Studying cell of origin is obviously more feasible in mice. The identification of markers for neural stem cells has enabled testing whether neural precursor compartments are more easily transformable than differentiated cell compartments. One problem remains that no single marker can precisely distinguish stem cells from progenitor cells, so that any cell compartment manipulation at best distinguishes only precursor cells from differentiated cells. Nestin and Sox2 mark stem cells as well as progenitor cells, but are the best characterized neural precursor markers described thus far. Some additional precursor markers become even more confusing, so for example glial fibrillary acidic protein (GFAP) seems to mark differentiated astrocytes as well as neural precursor cells, and its promoter has been formerly used to target gene alterations to differentiated cells, but more recently to precursors based on the identification of the Type B GFAP+ neural precursors in the subventricular zone of the adult mouse brain (Doetsch et al., 1999, 1997).
Despite these limitations, targeting precursor cells with oncogenic lesions in animals in vivo point to precursor cells or zones in the brain for being more easily initiated into brain tumors than non‐precursor zones. Holland's group was the first to show that the more primitive Nestin+ cell compartment was more permissive to neoplastic transformation into glioblastoma than a GFAP+ compartment, in response to forced EGFRvIII expression in neonatal mice using the RCAS‐tva system (Holland et al., 1998). Nestin positive compartments can also be targeted to induce medulloblastomas in mice (Rao et al., 2004). Tumor phenotype depends in part on the oncogene that is overexpressed. Elegant work from Parada's group has suggested that, in response to targeted combined deletion of p53 and NF1, only GFAP+ SVZ precursor compartments can be initiated into glioblastomas (Alcantara Llaguno et al., 2009; Zhu et al., 2005). Cre adenoviral injection at 4–8 weeks of age into the SVZ, but not the cortex of NF1flox/flox; p53flox/flox mice, NF1flox/flox; p53flox/− mice, or NF1flox/flox; PTENflox/+ mice resulted in malignant astrocytoma formation (Alcantara Llaguno et al., 2009). In another study, the frequency of tumor initiation in response to oncogenic Ras carrying Cre‐loxP controlled lentiviral vectors injected into the brains of adult GFAP‐Cre mice was also much greater when the hippocampus or SVZ was targeted, compared to the cortex (Marumoto et al., 2009). Targeting a mutant p53 gene throughout the brain causes a selective accumulation of mutant p53 protein in Olig2+ precursors in the SVZ, resulting in their expansion and subsequent glioma formation (Wang et al., 2009b). Mice bearing conditional alleles of p53, pRB, and PTEN in different combinations, which are injected with adenoCre, only have tumors arise in precursor zones and not from mature peripheral astrocytes (Jacques et al., 2009), with also tumor phenotype depending on the molecular alteration.
In vitro induced transformation of astrocyte populations (with EGFRvIII and Ink4a/Arf deficiency) may also give rise to brain tumors in experimental models, but the astrocytes that are cultured in vitro may represent a more primitive population than those found in vivo (Bachoo et al., 2002). Interestingly though, utilizing the same model of oncogenic transformation, superimposed on Bmi1 deficiency, suggests that the most aggressive brain tumor phenotypes may arise in the astrocyte compartment and not in a stem cell compartment (Bruggeman et al., 2007).
Other more restricted progenitors may also give rise to gliomas in experimental models. Myelinating oligodendrocyte precursor cells, marked by CNPase (2′,3′‐cyclic nucleotide 3′ phosphodiesterase) expression can be transformed into gliomas in response to forced expression of PDGF‐B (Lindberg et al., 2009). Interestingly, Akt and K‐Ras were not able to induce tumors in this model.
For medulloblastomas, Wechsler‐Reya's group has recently demonstrated that in response to complete loss of function of Patched1, a GFAP+ stem cell compartment, or a Math1+ committed granule neuron progenitor compartment are both permissive to give rise to medulloblastomas (Yang et al., 2008). The data, together with work from Rowitch's lab (Schuller et al., 2008), suggests the tumor initiating cell phenotype may not be manifest until the cells go through a granule cell precursor stage, reminiscent of what is observed in experimental malignant peripheral nerve sheath tumors. In these models of peripheral nervous system tumors, genetic changes must occur in neural crest stem cells to give rise to these malignant tumors, but the cell that then propagates the tumor does not have a neural crest stem cell phenotype, but has the phenotype of more differentiated progeny, Schwann cells (Joseph et al., 2008; Yang et al., 2008; Zheng et al., 2008a). Recent work from Marino's group also supports that medulloblastomas in mice can arise from precursor cells of a non‐granule cell lineage as well as from those of a granule cell lineage, in response to adenoCre viral injection into mice with floxed Rb and p53 alleles (Sutter et al., 2010). It is hoped that discerning distinct cells of origin for given tumor phenotypes may have impact on understanding the biological aggressiveness and targets available for therapeutic intervention.
8. Conclusion
The field of brain cancer stem cells remains in its infancy. There is a great deal more study required to better understand brain tumor heterogeneity and hierarchy and many questions remain unanswered. It remains critical to keep assays rigorous and to relate research findings made in in vitro systems back to in vivo systems. The full relevance of experimentally generated tumors in mice to human tumors must be continually sought, and it is my view that both need to be studied in parallel. Studies on human tumors must continue as a high priority for the field, and greater numbers of samples need to be made available to researchers in order to determine how widespread initial research findings are applicable to a larger number of patients with the disease. However, the pace of knowledge in this field is rapid, offering new hope to patients who are desperate for better treatment.
Acknowledgements
Dr. Dirks laboratory is funded by the Canadian Cancer Society Research Institute, the Canadian Institutes for Health Research, the Ontario Institute for Cancer Research, the Terry Fox Foundation, the Hospital for Sick Children Foundation, and Jessica's Footprint Foundation.
Dirks Peter B., (2010), Brain tumor stem cells: The cancer stem cell hypothesis writ large, Molecular Oncology, 4, doi: 10.1016/j.molonc.2010.08.001.
References
- Comprehensive genomic characterization defines human glioblastoma genes and core pathways. Nature. 455, 1061–1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Hajj, M. , Wicha, M.S. , Benito-Hernandez, A. , Morrison, S.J. , Clarke, M.F. , 2003. From the cover: prospective identification of tumorigenic breast cancer cells. Proc. Natl. Acad. Sci. USA. 100, 3983–3988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alcantara Llaguno, S. , Chen, J. , Kwon, C.H. , Jackson, E.L. , Li, Y. , Burns, D.K. , Alvarez-Buylla, A. , Parada, L.F. , 2009. Malignant astrocytomas originate from neural stem/progenitor cells in a somatic tumor suppressor mouse model. Cancer Cell. 15, 45–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander, C.M. , Puchalski, J. , Klos, K.S. , Badders, N. , Ailles, L. , Kim, C.F. , Dirks, P. , Smalley, M.J. , 2009. Separating stem cells by flow cytometry: reducing variability for solid tissues. Cell Stem Cell. 5, 579–583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachoo, R.M. , Maher, E.A. , Ligon, K.L. , Sharpless, N.E. , Chan, S.S. , You, M.J. , Tang, Y. , DeFrances, J. , Stover, E. , Weissleder, R. , 2002. Epidermal growth factor receptor and Ink4a/Arf: convergent mechanisms governing terminal differentiation and transformation along the neural stem cell to astrocyte axis. Cancer Cell. 1, 269–277. [DOI] [PubMed] [Google Scholar]
- Bao, S. , Wu, Q. , Li, Z. , Sathornsumetee, S. , Wang, H. , McLendon, R.E. , Hjelmeland, A.B. , Rich, J.N. , 2008. Targeting cancer stem cells through L1CAM suppresses glioma growth. Cancer Res. 68, 6043–6048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bao, S. , Wu, Q. , McLendon, R.E. , Hao, Y. , Shi, Q. , Hjelmeland, A.B. , Dewhirst, M.W. , Bigner, D.D. , Rich, J.N. , 2006. Glioma stem cells promote radioresistance by preferential activation of the DNA damage response. Nature. 444, 756–760. [DOI] [PubMed] [Google Scholar]
- Bao, S. , Wu, Q. , Sathornsumetee, S. , Hao, Y. , Li, Z. , Hjelmeland, A.B. , Shi, Q. , McLendon, R.E. , Bigner, D.D. , Rich, J.N. , 2006. Stem cell-like glioma cells promote tumor angiogenesis through vascular endothelial growth factor. Cancer Res. 66, 7843–7848. [DOI] [PubMed] [Google Scholar]
- Bar, E.E. , Chaudhry, A. , Lin, A. , Fan, X. , Schreck, K. , Matsui, W. , Piccirillo, S. , Vescovi, A.L. , DiMeco, F. , Olivi, A. , 2007. Cyclopamine-mediated hedgehog pathway inhibition depletes stem-like cancer cells in glioblastoma. Stem Cells. 25, 2524–2533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barami, K. , Sloan, A.E. , Rojiani, A. , Schell, M.J. , Staller, A. , Brem, S. , 2009. Relationship of gliomas to the ventricular walls. J. Clin. Neurosci. 16, 195–201. [DOI] [PubMed] [Google Scholar]
- Beier, D. , Hau, P. , Proescholdt, M. , Lohmeier, A. , Wischhusen, J. , Oefner, P.J. , Aigner, L. , Brawanski, A. , Bogdahn, U. , Beier, C.P. , 2007. CD133(+) and CD133(−) glioblastoma-derived cancer stem cells show differential growth characteristics and molecular profiles. Cancer Res. 67, 4010–4015. [DOI] [PubMed] [Google Scholar]
- Beier, D. , Rohrl, S. , Pillai, D.R. , Schwarz, S. , Kunz-Schughart, L.A. , Leukel, P. , Proescholdt, M. , Brawanski, A. , Bogdahn, U. , Trampe-Kieslich, A. , 2008. Temozolomide preferentially depletes cancer stem cells in glioblastoma. Cancer Res. 68, 5706–5715. [DOI] [PubMed] [Google Scholar]
- Beier, D. , Wischhusen, J. , Dietmaier, W. , Hau, P. , Proescholdt, M. , Brawanski, A. , Bogdahn, U. , Beier, C.P. , 2008. CD133 expression and cancer stem cells predict prognosis in high-grade oligodendroglial tumors. Brain Pathol. 18, 370–377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bleau, A.M. , Hambardzumyan, D. , Ozawa, T. , Fomchenko, E.I. , Huse, J.T. , Brennan, C.W. , Holland, E.C. , 2009. PTEN/PI3K/Akt pathway regulates the side population phenotype and ABCG2 activity in glioma tumor stem-like cells. Cell Stem Cell. 4, 226–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borovski, T. , Verhoeff, J.J. , ten Cate, R. , Cameron, K. , de Vries, N.A. , van Tellingen, O. , Richel, D.J. , van Furth, W.R. , Medema, J.P. , Sprick, M.R. , 2009. Tumor microvasculature supports proliferation and expansion of glioma-propagating cells. Int. J. Cancer. 125, 1222–1230. [DOI] [PubMed] [Google Scholar]
- Bruggeman, S.W. , Hulsman, D. , Tanger, E. , Buckle, T. , Blom, M. , Zevenhoven, J. , van Tellingen, O. , van Lohuizen, M. , 2007. Bmi1 controls tumor development in an Ink4a/Arf-independent manner in a mouse model for glioma. Cancer Cell. 12, 328–341. [DOI] [PubMed] [Google Scholar]
- Calabrese, C. , Poppleton, H. , Kocak, M. , Hogg, T.L. , Fuller, C. , Hamner, B. , Oh, E.Y. , Gaber, M.W. , Finklestein, D. , Allen, M. , 2007. A perivascular niche for brain tumor stem cells. Cancer Cell. 11, 69–82. [DOI] [PubMed] [Google Scholar]
- Campos, L.S. , Leone, D.P. , Relvas, J.B. , Brakebusch, C. , Fassler, R. , Suter, U. , ffrench-Constant, C. , 2004. Beta1 integrins activate a MAPK signalling pathway in neural stem cells that contributes to their maintenance. Development. 131, 3433–3444. [DOI] [PubMed] [Google Scholar]
- Capela, A. , Temple, S. , 2002. LeX/ssea-1 is expressed by adult mouse CNS stem cells, identifying them as nonependymal. Neuron. 35, 865–875. [DOI] [PubMed] [Google Scholar]
- Capela, A. , Temple, S. , 2006. LeX is expressed by principle progenitor cells in the embryonic nervous system, is secreted into their environment and binds Wnt-1. Dev. Biol. 291, 300–313. [DOI] [PubMed] [Google Scholar]
- Chen, R. , Nishimura, M.C. , Bumbaca, S.M. , Kharbanda, S. , Forrest, W.F. , Kasman, I.M. , Greve, J.M. , Soriano, R.H. , Gilmour, L.L. , Rivers, C.S. , 2010. A hierarchy of self-renewing tumor-initiating cell types in glioblastoma. Cancer Cell. 17, 362–375. [DOI] [PubMed] [Google Scholar]
- Clement, V. , Dutoit, V. , Marino, D. , Dietrich, P.Y. , Radovanovic, I. , 2009. Limits of CD133 as a marker of glioma self-renewing cells. Int. J. Cancer. 125, 244–248. [DOI] [PubMed] [Google Scholar]
- Clement, V. , Marino, D. , Cudalbu, C. , Hamou, M.F. , Mlynarik, V. , de Tribolet, N. , Dietrich, P.Y. , Gruetter, R. , Hegi, M.E. , Radovanovic, I. , 2010. Marker-independent identification of glioma-initiating cells. Nat. Methods. 7, 224–228. [DOI] [PubMed] [Google Scholar]
- Clement, V. , Sanchez, P. , de Tribolet, N. , Radovanovic, I. , Ruiz i Altaba, A. , 2007. HEDGEHOG-GLI1 signaling regulates human glioma growth, cancer stem cell self-renewal, and tumorigenicity. Curr. Biol. 17, 165–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conti, L. , Cattaneo, E. , 2010. Neural stem cell systems: physiological players or in vitro entities?. Nat. Rev. Neurosci. 11, 176–187. [DOI] [PubMed] [Google Scholar]
- Conti, L. , Pollard, S.M. , Gorba, T. , Reitano, E. , Toselli, M. , Biella, G. , Sun, Y. , Sanzone, S. , Ying, Q.L. , Cattaneo, E. , 2005. Niche-independent symmetrical self-renewal of a mammalian tissue stem cell. PLoS Biol. 3, e283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corti, S. , Locatelli, F. , Papadimitriou, D. , Donadoni, C. , Salani, S. , Del Bo, R. , Strazzer, S. , Bresolin, N. , Comi, G.P. , 2006. Identification of a primitive brain-derived neural stem cell population based on aldehyde dehydrogenase activity. Stem Cells. 24, 975–985. [DOI] [PubMed] [Google Scholar]
- Curtis, M.A. , Kam, M. , Nannmark, U. , Anderson, M.F. , Axell, M.Z. , Wikkelso, C. , Holtas, S. , van Roon-Mom, W.M. , Bjork-Eriksson, T. , Nordborg, C. , 2007. Human neuroblasts migrate to the olfactory bulb via a lateral ventricular extension. Science. 315, 1243–1249. [DOI] [PubMed] [Google Scholar]
- Deangelis, L.M. , 2010. Neuro-oncology: new hope for patients with gliomas. Lancet Neurol. 9, 17–18. [DOI] [PubMed] [Google Scholar]
- Diamandis, P. , Sacher, A.G. , Tyers, M. , Dirks, P.B. , 2009. New drugs for brain tumors? Insights from chemical probing of neural stem cells. Med. Hypotheses. 72, 683–687. [DOI] [PubMed] [Google Scholar]
- Diamandis, P. , Wildenhain, J. , Clarke, I.D. , Sacher, A.G. , Graham, J. , Bellows, D.S. , Ling, E.K. , Ward, R.J. , Jamieson, L.G. , Tyers, M. , 2007. Chemical genetics reveals a complex functional ground state of neural stem cells. Nat. Chem. Biol. 3, 268–273. [DOI] [PubMed] [Google Scholar]
- Dirks, P.B. , 2008. Brain tumor stem cells: bringing order to the chaos of brain cancer. J. Clin. Oncol. 26, 2916–2924. [DOI] [PubMed] [Google Scholar]
- Dirks, P.B. , 2008. Brain tumour stem cells: the undercurrents of human brain cancer and their relationship to neural stem cells. Philos. Trans. R Soc. Lond. B Biol. Sci. 363, 139–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doetsch, F. , Caille, I. , Lim, D.A. , Garcia-Verdugo, J.M. , Alvarez-Buylla, A. , 1999. Subventricular zone astrocytes are neural stem cells in the adult mammalian brain. Cell. 97, 703–716. [DOI] [PubMed] [Google Scholar]
- Doetsch, F. , Garcia-Verdugo, J.M. , Alvarez-Buylla, A. , 1997. Cellular composition and three-dimensional organization of the subventricular germinal zone in the adult mammalian brain. J. Neurosci. 17, 5046–5061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eyler, C.E. , Foo, W.C. , LaFiura, K.M. , McLendon, R.E. , Hjelmeland, A.B. , Rich, J.N. , 2008. Brain cancer stem cells display preferential sensitivity to Akt inhibition. Stem Cells. 26, 3027–3036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fael Al-Mayhani, T.M. , Ball, S.L. , Zhao, J.W. , Fawcett, J. , Ichimura, K. , Collins, P.V. , Watts, C. , 2009. An efficient method for derivation and propagation of glioblastoma cell lines that conserves the molecular profile of their original tumours. J. Neurosci. Methods. 176, 192–199. [DOI] [PubMed] [Google Scholar]
- Fan, X. , Khaki, L. , Zhu, T.S. , Soules, M.E. , Talsma, C.E. , Gul, N. , Koh, C. , Zhang, J. , Li, Y.M. , Maciaczyk, J. , 2009. NOTCH pathway blockade depletes CD133-positive glioblastoma cells and inhibits growth of tumor neurospheres and xenografts. Stem Cells. 28, 5–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan, X. , Matsui, W. , Khaki, L. , Stearns, D. , Chun, J. , Li, Y.M. , Eberhart, C.G. , 2006. Notch pathway inhibition depletes stem-like cells and blocks engraftment in embryonal brain tumors. Cancer Res. 66, 7445–7452. [DOI] [PubMed] [Google Scholar]
- Galli, R. , Binda, E. , Orfanelli, U. , Cipelletti, B. , Gritti, A. , De Vitis, S. , Fiocco, R. , Foroni, C. , Dimeco, F. , Vescovi, A. , 2004. Isolation and characterization of tumorigenic, stem-like neural precursors from human glioblastoma. Cancer Res. 64, 7011–7021. [DOI] [PubMed] [Google Scholar]
- Gil-Perotin, S. , Marin-Husstege, M. , Li, J. , Soriano-Navarro, M. , Zindy, F. , Roussel, M.F. , Garcia-Verdugo, J.M. , Casaccia-Bonnefil, P. , 2006. Loss of p53 induces changes in the behavior of subventricular zone cells: implication for the genesis of glial tumors. J. Neurosci. 26, 1107–1116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbertson, R.J. , Rich, J.N. , 2007. Making a tumour's bed: glioblastoma stem cells and the vascular niche. Nat. Rev. Cancer. 7, 733–736. [DOI] [PubMed] [Google Scholar]
- Groszer, M. , Erickson, R. , Scripture-Adams, D.D. , Dougherty, J.D. , Le Belle, J. , Zack, J.A. , Geschwind, D.H. , Liu, X. , Kornblum, H.I. , Wu, H. , 2006. PTEN negatively regulates neural stem cell self-renewal by modulating G0–G1 cell cycle entry. Proc. Natl. Acad. Sci. USA. 103, 111–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groszer, M. , Erickson, R. , Scripture-Adams, D.D. , Lesche, R. , Trumpp, A. , Zack, J.A. , Kornblum, H.I. , Liu, X. , Wu, H. , 2001. Negative regulation of neural stem/progenitor cell proliferation by the Pten tumor suppressor gene in vivo. Science. 294, 2186–2189. [DOI] [PubMed] [Google Scholar]
- Hall, P.E. , Lathia, J.D. , Caldwell, M.A. , Ffrench-Constant, C. , 2008. Laminin enhances the growth of human neural stem cells in defined culture media. BMC Neurosci. 9, 71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris, M.A. , Yang, H. , Low, B.E. , Mukherjee, J. , Guha, A. , Bronson, R.T. , Shultz, L.D. , Israel, M.A. , Yun, K. , 2008. Cancer stem cells are enriched in the side population cells in a mouse model of glioma. Cancer Res. 68, 10051–10059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hemmati, H.D. , Nakano, I. , Lazareff, J.A. , Masterman-Smith, M. , Geschwind, D.H. , Bronner-Fraser, M. , Kornblum, H.I. , 2003. Cancerous stem cells can arise from pediatric brain tumors. Proc. Natl. Acad. Sci. USA. 100, 15178–15183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holland, E.C. , Hively, W.P. , DePinho, R.A. , Varmus, H.E. , 1998. A constitutively active epidermal growth factor receptor cooperates with disruption of G1 cell-cycle arrest pathways to induce glioma-like lesions in mice. Genes Dev. 12, 3675–3685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huse, J.T. , Holland, E.C. , 2010. Targeting brain cancer: advances in the molecular pathology of malignant glioma and medulloblastoma. Nat. Rev. Cancer. 10, 319–331. [DOI] [PubMed] [Google Scholar]
- Ignatova, T.N. , Kukekov, V.G. , Laywell, E.D. , Suslov, O.N. , Vrionis, F.D. , Steindler, D.A. , 2002. Human cortical glial tumors contain neural stem-like cells expressing astroglial and neuronal markers in vitro. Glia. 39, 193–206. [DOI] [PubMed] [Google Scholar]
- Ikushima, H. , Todo, T. , Ino, Y. , Takahashi, M. , Miyazawa, K. , Miyazono, K. , 2009. Autocrine TGF-beta signaling maintains tumorigenicity of glioma-initiating cells through Sry-related HMG-box factors. Cell Stem Cell. 5, 504–514. [DOI] [PubMed] [Google Scholar]
- Iwamoto, F.M. , Abrey, L.E. , Beal, K. , Gutin, P.H. , Rosenblum, M.K. , Reuter, V.E. , DeAngelis, L.M. , Lassman, A.B. , 2009. Patterns of relapse and prognosis after bevacizumab failure in recurrent glioblastoma. Neurology. 73, 1200–1206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacques, T.S. , Swales, A. , Brzozowski, M.J. , Henriquez, N.V. , Linehan, J.M. , Mirzadeh, Z. , Catherine, O. Malley , Naumann, H. , Alvarez-Buylla, A. , Brandner, S. , 2009. Combinations of genetic mutations in the adult neural stem cell compartment determine brain tumour phenotypes. EMBO J. 29, 222–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joo, K.M. , Kim, S.Y. , Jin, X. , Song, S.Y. , Kong, D.S. , Lee, J.I. , Jeon, J.W. , Kim, M.H. , Kang, B.G. , Jung, Y. , 2008. Clinical and biological implications of CD133-positive and CD133-negative cells in glioblastomas. Lab. Invest. 88, 808–815. [DOI] [PubMed] [Google Scholar]
- Joseph, N.M. , Mosher, J.T. , Buchstaller, J. , Snider, P. , McKeever, P.E. , Lim, M. , Conway, S.J. , Parada, L.F. , Zhu, Y. , Morrison, S.J. , 2008. The loss of Nf1 transiently promotes self-renewal but not tumorigenesis by neural crest stem cells. Cancer Cell. 13, 129–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krivtsov, A.V. , Twomey, D. , Feng, Z. , Stubbs, M.C. , Wang, Y. , Faber, J. , Levine, J.E. , Wang, J. , Hahn, W.C. , Gilliland, D.G. , 2006. Transformation from committed progenitor to leukaemia stem cell initiated by MLL-AF9. Nature. 442, 818–822. [DOI] [PubMed] [Google Scholar]
- Laks, D.R. , Masterman-Smith, M. , Visnyei, K. , Angenieux, B. , Orozco, N.M. , Foran, I. , Yong, W.H. , Vinters, H.V. , Liau, L.M. , Lazareff, J.A. , 2009. Neurosphere formation is an independent predictor of clinical outcome in malignant glioma. Stem Cells. 27, 980–987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lathia, J.D. , Gallagher, J. , Heddleston, J.M. , Wang, J. , Eyler, C.E. , Macswords, J. , Wu, Q. , Vasanji, A. , McLendon, R.E. , Hjelmeland, A.B. , 2010. Integrin alpha 6 regulates glioblastoma stem cells. Cell Stem Cell. 6, 421–432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lathia, J.D. , Patton, B. , Eckley, D.M. , Magnus, T. , Mughal, M.R. , Sasaki, T. , Caldwell, M.A. , Rao, M.S. , Mattson, M.P. , ffrench-Constant, C. , 2007. Patterns of laminins and integrins in the embryonic ventricular zone of the CNS. J. Comp. Neurol. 505, 630–643. [DOI] [PubMed] [Google Scholar]
- Lee, J. , Kotliarova, S. , Kotliarov, Y. , Li, A. , Su, Q. , Donin, N.M. , Pastorino, S. , Purow, B.W. , Christopher, N. , Zhang, W. , 2006. Tumor stem cells derived from glioblastomas cultured in bFGF and EGF more closely mirror the phenotype and genotype of primary tumors than do serum-cultured cell lines. Cancer Cell. 9, 391–403. [DOI] [PubMed] [Google Scholar]
- Lee, J. , Son, M.J. , Woolard, K. , Donin, N.M. , Li, A. , Cheng, C.H. , Kotliarova, S. , Kotliarov, Y. , Walling, J. , Ahn, S. , 2008. Epigenetic-mediated dysfunction of the bone morphogenetic protein pathway inhibits differentiation of glioblastoma-initiating cells. Cancer Cell. 13, 69–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leone, D.P. , Relvas, J.B. , Campos, L.S. , Hemmi, S. , Brakebusch, C. , Fassler, R. , Ffrench-Constant, C. , Suter, U. , 2005. Regulation of neural progenitor proliferation and survival by beta1 integrins. J. Cell Sci. 118, 2589–2599. [DOI] [PubMed] [Google Scholar]
- Li, Z. , Bao, S. , Wu, Q. , Wang, H. , Eyler, C. , Sathornsumetee, S. , Shi, Q. , Cao, Y. , Lathia, J. , McLendon, R.E. , 2009. Hypoxia-inducible factors regulate tumorigenic capacity of glioma stem cells. Cancer Cell. 15, 501–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindberg, N. , Kastemar, M. , Olofsson, T. , Smits, A. , Uhrbom, L. , 2009. Oligodendrocyte progenitor cells can act as cell of origin for experimental glioma. Oncogene. 28, 2266–2275. [DOI] [PubMed] [Google Scholar]
- Liu, G. , Yuan, X. , Zeng, Z. , Tunici, P. , Ng, H. , Abdulkadir, I.R. , Lu, L. , Irvin, D. , Black, K.L. , Yu, J.S. , 2006. Analysis of gene expression and chemoresistance of CD133+ cancer stem cells in glioblastoma. Mol. Cancer. 5, 67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loulier, K. , Lathia, J.D. , Marthiens, V. , Relucio, J. , Mughal, M.R. , Tang, S.C. , Coksaygan, T. , Hall, P.E. , Chigurupati, S. , Patton, B. , 2009. beta1 integrin maintains integrity of the embryonic neocortical stem cell niche. PLoS Biol. 7, e1000176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marumoto, T. , Tashiro, A. , Friedmann-Morvinski, D. , Scadeng, M. , Soda, Y. , Gage, F.H. , Verma, I.M. , 2009. Development of a novel mouse glioma model using lentiviral vectors. Nat. Med. 15, 110–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meletis, K. , Wirta, V. , Hede, S.M. , Nister, M. , Lundeberg, J. , Frisen, J. , 2006. p53 suppresses the self-renewal of adult neural stem cells. Development. 133, 363–369. [DOI] [PubMed] [Google Scholar]
- Miraglia, S. , Godfrey, W. , Yin, A.H. , Atkins, K. , Warnke, R. , Holden, J.T. , Bray, R.A. , Waller, E.K. , Buck, D.W. , 1997. A novel five-transmembrane hematopoietic stem cell antigen: isolation, characterization, and molecular cloning. Blood. 90, 5013–5021. [PubMed] [Google Scholar]
- Molofsky, A.V. , Slutsky, S.G. , Joseph, N.M. , He, S. , Pardal, R. , Krishnamurthy, J. , Sharpless, N.E. , Morrison, S.J. , 2006. Increasing p16INK4a expression decreases forebrain progenitors and neurogenesis during ageing. Nature. 443, 448–452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakano, I. , Masterman-Smith, M. , Saigusa, K. , Paucar, A.A. , Horvath, S. , Shoemaker, L. , Watanabe, M. , Negro, A. , Bajpai, R. , Howes, A. , 2008. Maternal embryonic leucine zipper kinase is a key regulator of the proliferation of malignant brain tumors, including brain tumor stem cells. J. Neurosci. Res. 86, 48–60. [DOI] [PubMed] [Google Scholar]
- Ogden, A.T. , Waziri, A.E. , Lochhead, R.A. , Fusco, D. , Lopez, K. , Ellis, J.A. , Kang, J. , Assanah, M. , McKhann, G.M. , Sisti, M.B. , 2008. Identification of A2B5+CD133- tumor-initiating cells in adult human gliomas. Neurosurgery. 62, 505–514. discussion 514–505 [DOI] [PubMed] [Google Scholar]
- Pallini, R. , Ricci-Vitiani, L. , Banna, G.L. , Signore, M. , Lombardi, D. , Todaro, M. , Stassi, G. , Martini, M. , Maira, G. , Larocca, L.M. , 2008. Cancer stem cell analysis and clinical outcome in patients with glioblastoma multiforme. Clin. Cancer Res. 14, 8205–8212. [DOI] [PubMed] [Google Scholar]
- Pardal, R. , Clarke, M.F. , Morrison, S.J. , 2003. Applying the principles of stem-cell biology to cancer. Nat. Rev. Cancer. 3, 895–902. [DOI] [PubMed] [Google Scholar]
- Parsons, D.W. , Jones, S. , Zhang, X. , Lin, J.C. , Leary, R.J. , Angenendt, P. , Mankoo, P. , Carter, H. , Siu, I.M. , Gallia, G.L. , 2008. An integrated genomic analysis of human glioblastoma multiforme. Science. 321, 1807–1812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulino, A.C. , Teh, B.S. , 2005. Treatment of brain tumors. N. Engl. J. Med. 352, 2350–2353. author reply 2350–2353 [DOI] [PubMed] [Google Scholar]
- Penuelas, S. , Anido, J. , Prieto-Sanchez, R.M. , Folch, G. , Barba, I. , Cuartas, I. , Garcia-Dorado, D. , Poca, M.A. , Sahuquillo, J. , Baselga, J. , 2009. TGF-beta increases glioma-initiating cell self-renewal through the induction of LIF in human glioblastoma. Cancer Cell. 15, 315–327. [DOI] [PubMed] [Google Scholar]
- Perez-Losada, J. , Balmain, A. , 2003. Stem-cell hierarchy in skin cancer. Nat. Rev. Cancer. 3, 434–443. [DOI] [PubMed] [Google Scholar]
- Piccirillo, S.G. , Reynolds, B.A. , Zanetti, N. , Lamorte, G. , Binda, E. , Broggi, G. , Brem, H. , Olivi, A. , Dimeco, F. , Vescovi, A.L. , 2006. Bone morphogenetic proteins inhibit the tumorigenic potential of human brain tumour-initiating cells. Nature. 444, 761–765. [DOI] [PubMed] [Google Scholar]
- Pollard, S. , Clarke, I. , Smith, A. , Dirks, P. , 2009. Brain tumor stem cells: a level playing field. Cell Stem Cell. 5, 468–469. [Google Scholar]
- Pollard, S.M. , Conti, L. , Sun, Y. , Goffredo, D. , Smith, A. , 2006. Adherent neural stem (NS) cells from fetal and adult forebrain. Cereb. Cortex. 16, (Suppl. 1) i112–i120. [DOI] [PubMed] [Google Scholar]
- Pollard, S.M. , Yoshikawa, K. , Clarke, I.D. , Danovi, D. , Stricker, S. , Russell, R. , Bayani, J. , Head, R. , Lee, M. , Bernstein, M. , 2009. Glioma stem cell lines expanded in adherent culture have tumor-specific phenotypes and are suitable for chemical and genetic screens. Cell Stem Cell. 4, 568–580. [DOI] [PubMed] [Google Scholar]
- Rao, G. , Pedone, C.A. , Del Valle, L. , Reiss, K. , Holland, E.C. , Fults, D.W. , 2004. Sonic hedgehog and insulin-like growth factor signaling synergize to induce medulloblastoma formation from nestin-expressing neural progenitors in mice. Oncogene. 23, 6156–6162. [DOI] [PubMed] [Google Scholar]
- Read, T.A. , Fogarty, M.P. , Markant, S.L. , McLendon, R.E. , Wei, Z. , Ellison, D.W. , Febbo, P.G. , Wechsler-Reya, R.J. , 2009. Identification of CD15 as a marker for tumor-propagating cells in a mouse model of medulloblastoma. Cancer Cell. 15, 135–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rebetz, J. , Tian, D. , Persson, A. , Widegren, B. , Salford, L.G. , Englund, E. , Gisselsson, D. , Fan, X. , 2008. Glial progenitor-like phenotype in low-grade glioma and enhanced CD133-expression and neuronal lineage differentiation potential in high-grade glioma. PLoS One. 3, e1936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reya, T. , Morrison, S.J. , Clarke, M.F. , Weissman, I.L. , 2001. Stem cells, cancer, and cancer stem cells. Nature. 414, 105–111. [DOI] [PubMed] [Google Scholar]
- Reynolds, B.A. , Vescovi, A.L. , 2009. Brain cancer stem cells: Think twice before going flat. Cell Stem Cell. 5, 466–467. author reply 468–469 [DOI] [PubMed] [Google Scholar]
- Reynolds, B.A. , Weiss, S. , 1992. Generation of neurons and astrocytes from isolated cells of the adult mammalian central nervous system [see comments]. Science. 255, 1707–1710. [DOI] [PubMed] [Google Scholar]
- Russell, D.S. , Rubenstein, L.J. , 1989. Pathology of Tumours of the Nervous System fifth ed. Baltimore, Williams and Wilkins; [Google Scholar]
- Sanai, N. , Tramontin, A.D. , Quinones-Hinojosa, A. , Barbaro, N.M. , Gupta, N. , Kunwar, S. , Lawton, M.T. , McDermott, M.W. , Parsa, A.T. , Manuel-Garcia Verdugo, J. , 2004. Unique astrocyte ribbon in adult human brain contains neural stem cells but lacks chain migration. Nature. 427, 740–744. [DOI] [PubMed] [Google Scholar]
- Saxe, J.P. , Wu, H. , Kelly, T.K. , Phelps, M.E. , Sun, Y.E. , Kornblum, H.I. , Huang, J. , 2007. A phenotypic small-molecule screen identifies an orphan ligand-receptor pair that regulates neural stem cell differentiation. Chem. Biol. 14, 1019–1030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuller, U. , Heine, V.M. , Mao, J. , Kho, A.T. , Dillon, A.K. , Han, Y.G. , Huillard, E. , Sun, T. , Ligon, A.H. , Qian, Y. , 2008. Acquisition of granule neuron precursor identity is a critical determinant of progenitor cell competence to form Shh-induced medulloblastoma. Cancer Cell. 14, 123–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen, Q. , Goderie, S.K. , Jin, L. , Karanth, N. , Sun, Y. , Abramova, N. , Vincent, P. , Pumiglia, K. , Temple, S. , 2004. Endothelial cells stimulate self-renewal and expand neurogenesis of neural stem cells. Science. 304, 1338–1340. [DOI] [PubMed] [Google Scholar]
- Shen, Q. , Wang, Y. , Kokovay, E. , Lin, G. , Chuang, S.M. , Goderie, S.K. , Roysam, B. , Temple, S. , 2008. Adult SVZ stem cells lie in a vascular niche: a quantitative analysis of niche cell-cell interactions. Cell Stem Cell. 3, 289–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh, S.K. , Clarke, I.D. , Terasaki, M. , Bonn, V.E. , Hawkins, C. , Squire, J. , Dirks, P.B. , 2003. Identification of a cancer stem cell in human brain tumors. Cancer Res. 63, 5821–5828. [PubMed] [Google Scholar]
- Singh, S.K. , Hawkins, C. , Clarke, I.D. , Squire, J.A. , Bayani, J. , Hide, T. , Henkelman, R.M. , Cusimano, M.D. , Dirks, P.B. , 2004. Identification of human brain tumour initiating cells. Nature. 432, 396–401. [DOI] [PubMed] [Google Scholar]
- Son, M.J. , Woolard, K. , Nam, D.H. , Lee, J. , Fine, H.A. , 2009. SSEA-1 is an enrichment marker for tumor-initiating cells in human glioblastoma. Cell Stem Cell. 4, 440–452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stupp, R. , Mason, W.P. , van den Bent, M.J. , Weller, M. , Fisher, B. , Taphoorn, M.J. , Belanger, K. , Brandes, A.A. , Marosi, C. , Bogdahn, U. , 2005. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N. Engl. J. Med. 352, 987–996. [DOI] [PubMed] [Google Scholar]
- Sun, Y. , Kong, W. , Falk, A. , Hu, J. , Zhou, L. , Pollard, S. , Smith, A. , 2009. CD133 (Prominin) negative human neural stem cells are clonogenic and tripotent. PLoS One. 4, e5498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutter, R. , Shakhova, O. , Bhagat, H. , Behesti, H. , Sutter, C. , Penkar, S. , Santuccione, A. , Bernays, R. , Heppner, F.L. , Schuller, U. , 2010. Cerebellar stem cells act as medulloblastoma-initiating cells in a mouse model and a neural stem cell signature characterizes a subset of human medulloblastomas. Oncogene. 29, 1845–1856. [DOI] [PubMed] [Google Scholar]
- Tamase, A. , Muraguchi, T. , Naka, K. , Tanaka, S. , Kinoshita, M. , Hoshii, T. , Ohmura, M. , Shugo, H. , Ooshio, T. , Nakada, M. , 2009. Identification of tumor-initiating cells in a highly aggressive brain tumor using promoter activity of nucleostemin. Proc. Natl. Acad. Sci. USA. 106, 17163–17168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tavazoie, M. , Van der Veken, L. , Silva-Vargas, V. , Louissaint, M. , Colonna, L. , Zaidi, B. , Garcia-Verdugo, J.M. , Doetsch, F. , 2008. A specialized vascular niche for adult neural stem cells. Cell Stem Cell. 3, 279–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tchoghandjian, A. , Baeza, N. , Colin, C. , Cayre, M. , Metellus, P. , Beclin, C. , Ouafik, L. , Figarella-Branger, D. , 2010. A2B5 cells from human glioblastoma have cancer stem cell properties. Brain Pathol. 20, 211–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thon, N. , Damianoff, K. , Hegermann, J. , Grau, S. , Krebs, B. , Schnell, O. , Tonn, J.C. , Goldbrunner, R. , 2010. Presence of pluripotent CD133+ cells correlates with malignancy of gliomas. Mol. Cell Neurosci. 43, 51–59. [DOI] [PubMed] [Google Scholar]
- Uchida, N. , Buck, D.W. , He, D. , Reitsma, M.J. , Masek, M. , Phan, T.V. , Tsukamoto, A.S. , Gage, F.H. , Weissman, I.L. , 2000. Direct isolation of human central nervous system stem cells. Proc. Natl. Acad. Sci. USA. 97, 14720–14725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Visvader, J.E. , Lindeman, G.J. , 2008. Cancer stem cells in solid tumours: accumulating evidence and unresolved questions. Nat. Rev. Cancer. 8, 755–768. [DOI] [PubMed] [Google Scholar]
- Wang, J. , Sakariassen, P.O. , Tsinkalovsky, O. , Immervoll, H. , Boe, S.O. , Svendsen, A. , Prestegarden, L. , Rosland, G. , Thorsen, F. , Stuhr, L. , 2008. CD133 negative glioma cells form tumors in nude rats and give rise to CD133 positive cells. Int. J. Cancer. 122, 761–768. [DOI] [PubMed] [Google Scholar]
- Wang, J. , Wakeman, T.P. , Lathia, J.D. , Hjelmeland, A.B. , Wang, X.F. , White, R.R. , Rich, J.N. , Sullenger, B.A. , 2009. Notch promotes radioresistance of glioma stem cells. Stem Cells. 28, 17–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, J. , Wang, H. , Li, Z. , Wu, Q. , Lathia, J.D. , McLendon, R.E. , Hjelmeland, A.B. , Rich, J.N. , 2008. c-Myc is required for maintenance of glioma cancer stem cells. PLoS One. 3, e3769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, Y. , Yang, J. , Zheng, H. , Tomasek, G.J. , Zhang, P. , McKeever, P.E. , Lee, E.Y. , Zhu, Y. , 2009. Expression of mutant p53 proteins implicates a lineage relationship between neural stem cells and malignant astrocytic glioma in a murine model. Cancer Cell. 15, 514–526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward, R.J., Dirks, P.B., 2006. Cancer stem cells: at the headwaters of tumor development. [DOI] [PubMed]
- Ward, R.J. , Lee, L. , Graham, K. , Satkunendran, T. , Yoshikawa, K. , Ling, E. , Harper, L. , Austin, R. , Nieuwenhuis, E. , Clarke, I.D. , 2009. Multipotent CD15+ cancer stem cells in patched-1-deficient mouse medulloblastoma. Cancer Res. 69, 4682–4690. [DOI] [PubMed] [Google Scholar]
- Weigmann, A. , Corbeil, D. , Hellwig, A. , Huttner, W.B. , 1997. Prominin, a novel microvilli-specific polytopic membrane protein of the apical surface of epithelial cells, is targeted to plasmalemmal protrusions of non-epithelial cells. Proc. Natl. Acad. Sci. USA. 94, 12425–12430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen, P.Y. , Kesari, S. , 2008. Malignant gliomas in adults. N. Engl. J. Med. 359, 492–507. [DOI] [PubMed] [Google Scholar]
- Wurdak, H. , Zhu, S. , Romero, A. , Lorger, M. , Watson, J. , Chiang, C.Y. , Zhang, J. , Natu, V.S. , Lairson, L.L. , Walker, J.R. , 2010. An RNAi screen identifies TRRAP as a regulator of brain tumor-initiating cell differentiation. Cell Stem Cell. 6, 37–47. [DOI] [PubMed] [Google Scholar]
- Yang, Z.J. , Ellis, T. , Markant, S.L. , Read, T.A. , Kessler, J.D. , Bourboulas, M. , Schuller, U. , Machold, R. , Fishell, G. , Rowitch, D.H. , 2008. Medulloblastoma can be initiated by deletion of patched in lineage-restricted progenitors or stem cells. Cancer Cell. 14, 135–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin, A.H. , Miraglia, S. , Zanjani, E.D. , Almeida-Porada, G. , Ogawa, M. , Leary, A.G. , Olweus, J. , Kearney, J. , Buck, D.W. , 1997. AC133, a novel marker for human hematopoietic stem and progenitor cells. Blood. 90, 5002–5012. [PubMed] [Google Scholar]
- Zeppernick, F. , Ahmadi, R. , Campos, B. , Dictus, C. , Helmke, B.M. , Becker, N. , Lichter, P. , Unterberg, A. , Radlwimmer, B. , Herold-Mende, C.C. , 2008. Stem cell marker CD133 affects clinical outcome in glioma patients. Clin. Cancer Res. 14, 123–129. [DOI] [PubMed] [Google Scholar]
- Zheng, H. , Chang, L. , Patel, N. , Yang, J. , Lowe, L. , Burns, D.K. , Zhu, Y. , 2008. Induction of abnormal proliferation by nonmyelinating schwann cells triggers neurofibroma formation. Cancer Cell. 13, 117–128. [DOI] [PubMed] [Google Scholar]
- Zheng, H. , Ying, H. , Yan, H. , Kimmelman, A.C. , Hiller, D.J. , Chen, A.J. , Perry, S.R. , Tonon, G. , Chu, G.C. , Ding, Z. , 2008. p53 and Pten control neural and glioma stem/progenitor cell renewal and differentiation. Nature. 455, 1129–1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou, B.B. , Zhang, H. , Damelin, M. , Geles, K.G. , Grindley, J.C. , Dirks, P.B. , 2009. Tumour-initiating cells: challenges and opportunities for anticancer drug discovery. Nat. Rev. Drug Discov. 8, 806–823. [DOI] [PubMed] [Google Scholar]
- Zhu, Y. , Guignard, F. , Zhao, D. , Liu, L. , Burns, D.K. , Mason, R.P. , Messing, A. , Parada, L.F. , 2005. Early inactivation of p53 tumor suppressor gene cooperating with NF1 loss induces malignant astrocytoma. Cancer Cell. 8, 119–130. [DOI] [PMC free article] [PubMed] [Google Scholar]


