Abstract
Hyaluronan (HA) is a naturally occurring glycosaminoglycan widely researched for its use as a biomaterial in tissue engineering, drug delivery, angiogenesis, and ophthalmic surgeries. The mechanical properties of this biomaterial can be altered to a required extent by chemically modifying the pendant reactive groups. However, derivatizing these polymers to a predetermined extent has been the Achilles heel for this process. In this study, we have investigated the factors controlling the derivatization of the carboxyl moieties of HA with amine containing thiol, cystamine dihydrochloride (Cys), via carbodiimide crosslinking chemistry. We used fractional factorial design to screen and identify the significant factor(s) affecting the reaction, and response surface methodology (RSM) to develop a model equation for predicting the degree of thiolation of HA. Also, we analyzed the reaction mechanism for potential side reactions. We observed that, N-(3-Dimethylaminopropyl)-N′-ethylcarbodiimide hydrochloride (EDC) (mole ratio with repeat unit of HA) is the significant factor controlling the degree of amidation. The quadratic equations developed from RSM predict the formulation for a desired degree of amidation of HA and percentage of potential side product. Hence, derivatizing HA to a predetermined extent with minimal side product can be achieved using the statistical design of experiments.
Keywords: hyaluronan, thiol, carbodiimide crosslinking, response surface methodology, side reaction
INTRODUCTION
Hyaluronan (HA), a bioactive disaccharide polymer, is a linear non-sulfated glycosaminoglycan made with repeating units of D-glucuronic acid and N-acetyl-D-glucosamine linked via β-(1-3) and β-(1-4) glycosidic bonds. It is found in significant amounts in vitreous, synovial fluid of joints, extracellular matrix and connective tissues, where it plays a critical role in regulating cellular functions. HAs distinctive physicochemical properties and biological functions make it useful in the biomedical field and biotechnological products as a biomaterial.12 HA hydrogels show promising applications in tissue engineering and wound healing2 by facilitating cartilage repair, oriented bone regeneration, and sustained cell expansion. It is useful in ophthalmic surgeries3 as a vitreous substitute after vitrectomy, angiogenesis,4 targeted drug delivery and cell encapsulation5. Designing HA scaffolds specifically for use in tissue engineering and drug delivery is important, in that the mechanical properties of HA hydrogels, mainly the porosity, are altered by covalent chemical crosslinking of pendant reactive groups by addition/condensation chemistry or by radical polymerization.2,6 Recently, HA was modified with thiol cross-linking sites to form a stable hydrogel scaffold to examine its effects on in-vitro cortical cell growth.7 Eng7 showed that varying the degree of substitution of the HA altered its biological activity. Slightly modified HAs retain sufficient binding sites for HA receptors, appropriate for target-specific delivery. On the other hand, high modification of HA destroys much of its characteristics, enabling applications for conjugation of peptides and proteins as long acting therapeutics.8 Thiol-modified HAs are used for coating gold nanoparticles to prevent protein adsorption,9 opsonization,10 as well as nonspecific delivery of drugs. Chemical modifications of HAs are also critical to prevent their rapid degradation and their diffusion out of the vitreous cavity in applications as a permanent vitreous substitute.3
In our work, we investigate the modification of HA in which the carboxyl group, in one of the saccharide groups, is derivatized to an amide containing a thiol group. The derivatization is accomplished by the well-known carbodiimide-based activation11–13 of the carboxyl group of HA with N-(3-Dimethylaminopropyl)-N′-ethylcarbodiimide hydrochloride (EDC) and N-hydroxylsuccinimide (NHS), followed by its reaction with a disulfide-containing amine. 12–15,16,12,17,18–20,16,20,21Based on the details of experimental conditions from several published sources, we focused on identifying the relative effects of various reaction parameters or factors. Using the design of experiments (DoE) statistics, involving fractional factorial design (FF)14,15 and response surface methodology (RSM),16–18 one can effectively design experiments to quantitatively identify the relationship of significant factor(s) that affect the reaction output (response). This enables one to statistically optimize the reaction parameters to yield a desired output, carry out better trade-offs when multiple critical factors are governing the reaction, and minimize variability. In DoE involving multiple factors, a particular design is chosen based on the number of factors to be tested and the levels over which they vary. In general, the factors affecting the process are initially screened using fractional factorial designs to determine their significance. Two to four key factors are further optimized using RSM to maximize, minimize, or stabilize the response.
In this work, 22,27,28we have attempted to identify the relative importance of reaction variables or factors influencing the amidation reaction and determine the quantitative relationship between the factors and their responses. The five main factors we investigated, using FF design, were the pH, duration of reaction, and the mole ratios of Cys, EDC, and NHS with respect to a repeat unit of HA (disaccharide consisting of glucoronic acid and N-acetyl glucoseamine). The mole ratios of reactants to repeat unit of HA, will be mentioned as moles of reactant in this paper. The response was the degree of amidation of HA. We used a D-optimal design of RSM to determine how each factor affected the degree of thiolation of HA and the formation of side products. We optimized the reaction for a pre-determined extent of derivatization with minimal side product.
MATERIALS AND METHODS
Materials
Hyaluronic acid (MW 60 kDa) was purchased from Lifecore Biomedical (Chaska, MN). 1-ethyl-3-(3-dimethylaminopropyl) carbodiimide (EDC), N-hydroxysuccinimide (NHS), cystamine dihydrochloride (Cys), dithiotheritol (DTT), glycine, sodium sulphite, sodium hydroxide, sodium chloride, and ethylenediaminetetraacetic acid were purchased from Sigma Aldrich (St. Louis, MO). The FF and RSM experiments were designed using Design-Expert® software, version 7, Stat-Ease (Minneapolis, MN).
Design of Experiments
Selection of factors affecting the reaction
An essential step of planning the factorial process is selecting the factor(s) and their levels of variation. Five main factors affecting the degree of amidation of HA were moles of Cys, EDC and NHS; pH, and duration of the reaction. The other critical factor affecting the reaction was the temperature, which was kept constant at 37 °C. The factors were varied at two levels for FF and five levels for RSM.
Fractional factorial design for screening the significant factors
The experimental design and statistical analysis were carried out in Design-Expert 7.0.0. We chose a two-level fractional factorial design to investigate the factors and screen for the significant variables affecting the response. We chose a design of resolution V in which the main effects were un-confounded, while two-factor interactions were confounded with three-factor interactions [Table I] in 16 experimental runs (2(5−1)). We conducted the experiments in random order to nullify the effect of systematic errors.
TABLE I.
Alias structure of the 2(5−1) fractional-factorial design
| Effect | Factors |
|---|---|
| [A] | A |
| [B] | B |
| [C] | C |
| [D] | D |
| [E] | E |
| [AB] | AB+CDE |
| [AC] | AC+BDE |
| [AD] | AD+BCE |
| [AE] | AE+BCD |
| [BC] | BC+ADE |
| [BD] | BD+ACE |
| [BE] | BE+ACD |
| [CD] | CD+ABE |
| [CE] | CE+ABD |
| [DE] | DE+ABC |
D-Optimal design for optimizing the degree of amidation of HA
The relationships between the significant factors obtained from FF analysis, were determined by a two-factor, five-level D-Optimal design with a quadratic model of RSM. A second-order polynomial, Equation (1), was used to calculate the predicted response:
| (1) |
where Y is the predicted response, X1 and X2 are independent variables (factors), b0 is the intercept, b1 and b2 are linear, b11 and b22 are squared, and b12 are interaction effects of the factors. To investigate their effect over the responses, the degree of amidation of HA (Y), the factors, X1 and X2 were varied over 5 levels, with different combinations resulting from 16 experiments [Table III].
TABLE III.
Table representing the experimental conditions (factors) and thiol content (response) obtained from disulfide tests for each experiment using response-surface design
| Run | Factor A: EDC | Factor B: Amine | Response: Thiol Content |
|---|---|---|---|
| (moles) | (moles) | (in % of Derivatization of HA) | |
| 16 | 0.5 | 0.5 | 6.80 |
| 3 | 10 | 0.5 | 15.74 |
| 14 | 0.5 | 10 | 7.61 |
| 12 | 10 | 10 | 27.5 |
| 10 | 5.25 | 10 | 22.52 |
| 4 | 10 | 5.25 | 26.33 |
| 5 | 5.25 | 2.88 | 23.95 |
| 11 | 0.5 | 5.25 | 5.52 |
| 15 | 2.88 | 7.63 | 25.89 |
| 7 | 7.63 | 7.63 | 37.12 |
| 9 | 5.25 | 0.5 | 21.47 |
| 8 | 0.5 | 0.5 | 6.82 |
| 13 | 10 | 0.5 | 24.87 |
| 2 | 0.5 | 10 | 7.65 |
| 1 | 10 | 10 | 34.50 |
| 6 | 10 | 5.25 | 36.34 |
Amidation of HA
We did the FF as a single block design [Table II]. Stock solutions of HA (1% (w/v)); EDC, NHS, and Cys of respective moles at two levels of concentration and pH were prepared. The total reaction volume of the experiment was 5 mL. A required volume of HA repeat unit, EDC, NHS, and Cys was added to 0.5 M MES buffer and the mixture was maintained at the correct pH in a closed 20-mL glass vial. This was continuously agitated in a shaker at 200 rpm for a required duration at 37°C. The reaction was stopped by adding 1 N NaOH; the final pH was adjusted to 8–9. Excess EDC, NHS, and Cys were removed from the reaction mixture by dialyzing (MWCO: 6,000–8,000) in deionized (DI) H2O (thrice). The pH of samples was adjusted to 7.5 and reduced with DTT for 3 h. Excess DTT was removed by dialyzing (MWCO: 6,000–8,000) in N2-bubbled 1 mM HCl (six times). The samples were analyzed for the percentage of derivatization of HA with thiols, using a disulfide and an Ellman’s test.
TABLE II.
The experimental condition (factors) and thiol content (response) obtained from a disulfide test for each experiment using FF design
| Run | Factor A: Cystamine | Factor B: pH | Factor C: NHS | Factor D: EDC | Factor E: Time | Response: Thiol content |
|---|---|---|---|---|---|---|
| (moles) | (moles) | (moles) | (hours) | (in % of derivatization of HA) | ||
| 13 | 0.1 | 4.5 | 0.1 | 1 | 6 | 0.27 |
| 3 | 10 | 4.5 | 0.1 | 1 | 1 | 0.77 |
| 11 | 0.1 | 6.5 | 0.1 | 1 | 1 | 3.73 |
| 5 | 10 | 6.5 | 0.1 | 1 | 6 | 10.22 |
| 2 | 0.1 | 4.5 | 1 | 1 | 1 | 0.28 |
| 14 | 10 | 4.5 | 1 | 1 | 6 | 0.35 |
| 4 | 0.1 | 6.5 | 1 | 1 | 6 | 6.28 |
| 15 | 10 | 6.5 | 1 | 1 | 1 | 19.55 |
| 10 | 0.1 | 4.5 | 0.1 | 15 | 1 | 4.67 |
| 6 | 10 | 4.5 | 0.1 | 15 | 6 | 29.28 |
| 8 | 0.1 | 6.5 | 0.1 | 15 | 6 | 5.44 |
| 9 | 10 | 6.5 | 0.1 | 15 | 1 | 17.16 |
| 7 | 0.1 | 4.5 | 1 | 15 | 6 | 5.05 |
| 16 | 10 | 4.5 | 1 | 15 | 1 | 13.89 |
| 12 | 0.1 | 6.5 | 1 | 15 | 1 | 5.01 |
| 1 | 10 | 6.5 | 1 | 15 | 6 | 22.21 |
The 16 combinations of RSM were performed using the same procedure as specified in FF design.
Determination of degree of amidation via disulfide linkage
Using the 2-nitro-5-thiosulfobenzonate (NTSB) assay,19 we determined the total thiol and disulfide content of the derivatized HA before and after reducing with DTT. Briefly, Cys standards of concentrations from 0.05 to 1.5 mM were prepared using a 10 mM Cys stock by serial dilution, using N2-bubbled DI water. About 900 μL of NTSB solution, prepared as described in the literature,19 was added to 100 μL of the sample and the standards. Because the reactions are photo-activated, assay samples were incubated in the dark for 15 minutes and their absorbance was read with a spectrophotometer at 412 nm.
Ellman’s reaction
The amount of thiol content was also determined spectrophotometrically, using an Ellman’s reagent as described by Ellman.20 Briefly, we diluted 10 mg of each lyophilized sample with 2 mL of N2-bubbled water. To 100 μL of this sample, we added 500 μL of 0.1 M phosphate buffer (pH 8), 400 μL of water, and 50 μL of Ellman’s reagent. Samples were incubated for 15 min at room temperature and their absorbance measured at 412 nm in a spectrophotometer. Thiol content was calculated as per the equation:
| (2) |
where Ab is the absorbance and Vtotal is the total volume, while Vsample is the sample volume and 13600 is the extinction coefficient. The concentration of the HA (HA conc) is in mg/mL.
Characterization of Amidated HA
The amidated HA was characterized using 1H NMR spectroscopy. 1H NMR spectra were obtained on a Varian Unity Inova 500 (Palo Alto, CA). HA samples were dissolved in D2O (8 mg/mL) with NaOD (20 μmol). Each sample was scanned for 128 times at 25°C.
RESULTS AND DISCUSSION
Amidation and Characterization of HA
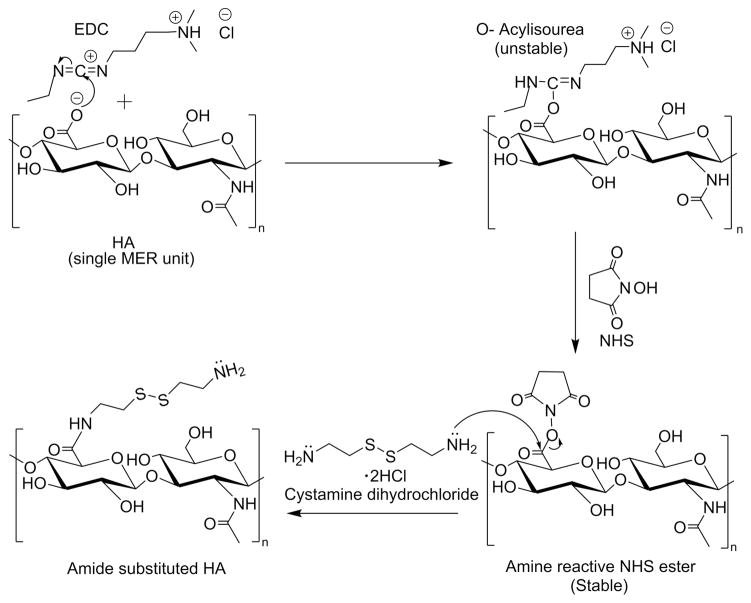
Derivatization of carboxylic moieties of HA, to an amide containing thiol group with cystamine dihydrochloride via carbodiimide crosslinking chemistry, proceeds as described in Fig 1. Mechanistically, the negatively charged carboxylate group attacks the electron-deficient diimide carbon atom on the carbodiimide molecule (EDC) to form the activated O-acylisourea intermediate. The result is that the carbon atom of the HA carboxylate group becomes sufficiently electron-deficient to be susceptible to nucleophilic attack by the lone pair of electrons on the amine group of the Cys, thereby forms an amide-substituted HA molecule and acylurea. The O-acylisourea, which is very unstable, is usually stabilized by substituting it with NHS while maintaining it in an active form.
FIGURE 1.
Carbodiimide crosslinking reaction of HA and Cys with EDC and NHS
Several investigators11–13,21 have derivatized HA through this mechanism, however, these procedures did not describe the quantitative relationship needed for optimization between the reactants and the degree of thiolation. Sehgal22 optimized the reaction conditions one factor at a time to link monovalent butyric acid over Affi-gel 102 with the use of EDC and NHS. These studies showed the significance of NHS for better yield of products, as well as the advantage of an MES buffer rather than a phosphate buffer. The experiments were carried out over a range of carboxylic acid:amine mole ratios with a maximum of 1:15; EDC:NHS mole ratios with a maximum of 15:1; pH of 3 to 8; and a duration of 1–24 h. Kafedjiiski11 focused on synthesizing a novel HA-cysteine ethyl ester conjugate mediated by EDC and NHS. The experiments were performed at pH 5.5 for 4 h with varying EDC:NHS ratios. Similarly, Cao23 analyzed the mechanical stability of collagen-chondroitin sulfate scaffolds with various EDC concentrations. The experiments were carried out for about 4 h in MES buffer for various concentrations of EDC with a constant EDC:NHS ratio of 4:1. In the literature, the duration of the crosslinking reaction has varied from 1 h to 24 h, with the majority being around 4 h.24–26 Sehgal22 observed that the percentage of coupling plateaued beyond 6 h. Most investigators used a pH of 4.5 to 6.5; while the majority used a pH of 5.26,27
We analyzed the dependence of these factors on one-another by designing experiments using FF design varying at two levels whose limits were chosen based on the literature [Table II]. The final product of the reaction, amide-substituted HA, was reduced with DTT, purified with DI water free of oxygen (through nitrogen bubbling) at acidic pH and lyophilized to obtain a white fluffy, fibrous solid. The degree of thiolation of the derivatized HA was determined using the disulfide test and Ellman’s test. The disulfide test measures both thiol and disulfide content in the sample and the Ellman’s test measures only the thiol content. The results from Ellman’s test are 30%–50% lower than the disulfide test results. This is because HA-SH is partially oxidized to disulfide the purification process. Hence, the disulfide test results were chosen for statistical analysis.
Fractional factorial design
Determination of significant factors from FF design
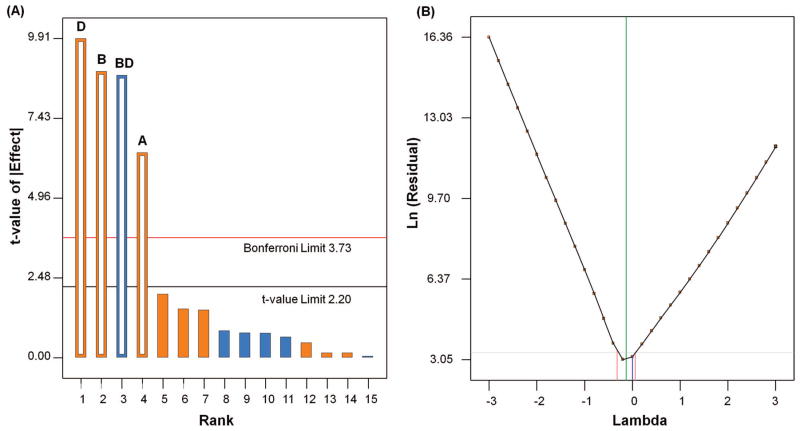
The response from the disulfide test for FF design [Table II] varied from a minimum of 0.27% to a maximum of 29.28% with a mean of 9.01% and standard deviation of 8.59%. We identified significant factors from the normal probability plot by choosing those factors that fall away from the standard line [Fig. 2]. The normal probability plot shows that the single factors EDC, the pH of the reaction medium, and Cys have a positive effect on the response, while the two-factor interaction between pH and EDC has a negative effect. Also, the Pareto diagram gives the contribution of each factor, which is useful in selecting the factors [Fig. 3(a)]. Having selected the important factors, we determined the need for transformation of data based on value of the ratio of maximum over minimum response. Generally, the response ratio above 10 may require a transformation, while a ratio below 3 may not need transformation. In our design, for a high response ratio of 108.4, we needed a log transformation, which is confirmed by the Box-Cox plot of power transforms [Fig. 3(b)].
FIGURE 2.
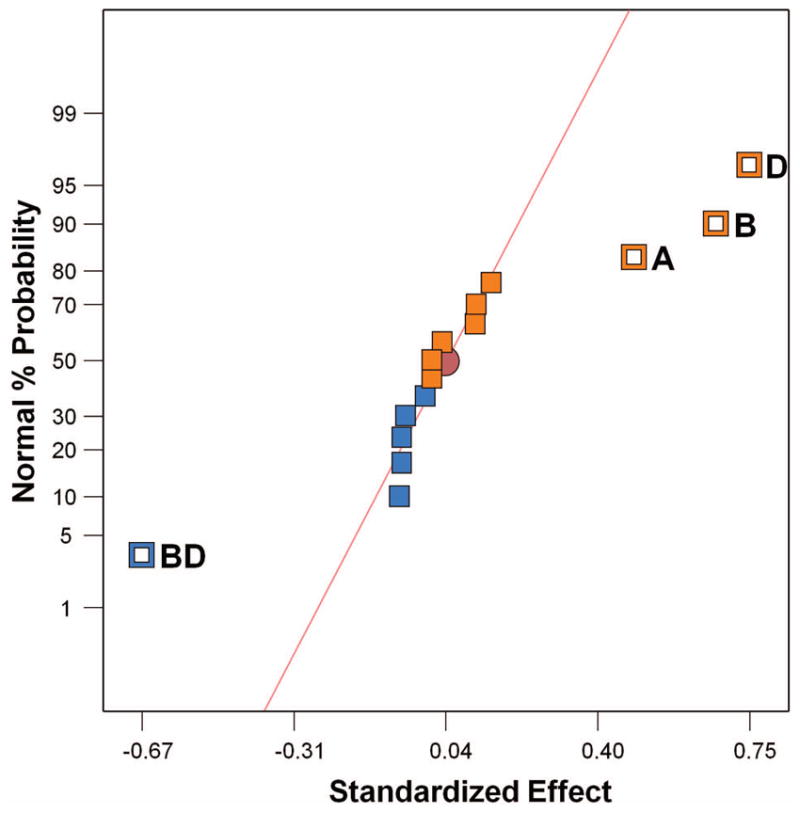
Normal probability plot of the effects of the factors and their interactions. Effect of single factors is represented by A, B, C, D, E; their interactions with other variables are represented as AB, BC, etc. In this figure, A- Cys; B- pH; D- EDC; BD- two factorial interaction of pH and EDC.
 represents positive effects; while
represents positive effects; while
 represents negative effects. The model is framed based on selecting the dominant factors that are highlighted and labeled in plots; other factors are considered as errors.
represents negative effects. The model is framed based on selecting the dominant factors that are highlighted and labeled in plots; other factors are considered as errors.
FIGURE 3.
(a) Pareto chart representing the effect of each factor over response variable in the descending order from greatest to lowest contribution for FF design. Bonferroni limit is the threshold where the effect emerging above this limit are significant. T limit is the threshold where the effect emerging above this but below Bonferroni limit may possibly be significant. Effects D, B, BD, A are significant. b) Box-Cox plot of power transforms representing the required transformation of data for FF design. In this figure,
 represents lambda current = 0;
represents lambda current = 0;
 is best lambda current = −0.13; recommeded transform is Log transformation with lambda = 0.
is best lambda current = −0.13; recommeded transform is Log transformation with lambda = 0.
The quality of the fitted model was evaluated by analysis of variance (ANOVA), a statistical method for making simultaneous comparisons between two or more means. It yields values that are analyzed for the existence of a significant relationship between the variables. In this study, we found the fitted model to be highly significant, with a determination coefficient (R2) of 0.96. Also, the predicted R2 of 0.92 was in reasonable agreement with the adjusted R2 of 0.95. The F-test assesses whether any of the effect of factors on response is, on average, superior or inferior to the others versus the null hypothesis, which considered all factors to yield the same mean response. The p value helps to assess if the main effect of each factor is statistically significant (p < 0.05) or marginally significant (p < 0.1).
The Model F value of 73.67 and p value of < 0.0001 indicates that the model is significant [Table IV]. There is only a 0.01% chance that a Model F-value this large could occur due to noise. Thus, the significant factors influencing the response from FF design are EDC, pH of the reaction medium, Cys, and interaction between EDC and pH.
TABLE IV.
Model Summary Statistics of the FF and RSM Models
| Models | F-value | p-value | R2 | Adjusted R2 | Predicted R2 | Adequate Precision |
|---|---|---|---|---|---|---|
| FF | 73.67 | < 0.0001 | 0.96 | 0.95 | 0.92 | 22.51 |
| RSM-Thiol Content | 39.43 | < 0.0001 | 0.91 | 0.88 | 0.84 | 14.79 |
| RSM-Side reaction | 56.09 | < 0.0001 | 0.92 | 0.90 | 0.87 | 14.37 |
Diagnostic plots of residuals
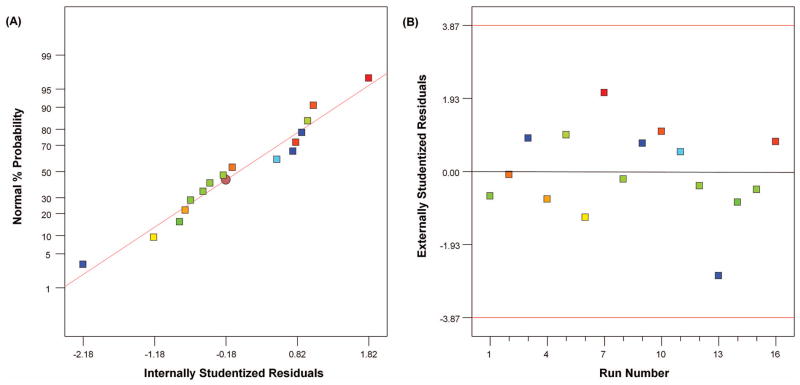
While the ANOVA emphasizes the significance of the chosen model of contributory factors over the response, the distribution of residuals, which are the difference between the predicted and observed values, is also a crucial part to be examined. The normal probability plot of residuals helps us assess how closely the observed values follow the theoretical distribution, which was indicated by a straight line showing normal distribution [Fig. 4(a)]. The externally studentized residual is the plot of residuals versus run, which tests whether the run in question is consistent with the rest of the data for this model. This helps to determine the outliers and whether there is a need to investigate them. For this model, the residuals are uniformly distributed for each run [Fig. 4(b)] and lie within the control limits, thereby indicating the run is consistent with the rest of the data.
FIGURE 4.
Residual analysis of the FF design. (a) Plot of normal probability of residuals and (b) Plot of external studentised residuals with each run.
 represents the gradient of colors with increasing Log10 (Thiol Content) from −0.57 to 1.47.
represents the gradient of colors with increasing Log10 (Thiol Content) from −0.57 to 1.47.
Plots of dominant effects on response
In this study, based on the ANOVA and residual analysis, it is evident that the EDC, pH of the reaction medium and Cys are the significant single factors governing the thiolation of HA. These factors show a positive effect in the specified range, indicating the higher degree of amidation near the maximum range of the factors. This is in agreement with the one factor analysis performed by Sehgal22 for optimizing the amidation reaction. However, FF provides aditional information on the interaction between the factors, which is not obtained through one-at-a-time factor analysis. We observed the effect of a two-factor interaction between EDC and the pH of the reaction medium to be significant. The graph for the interaction between the factors enables us to understand how the independent variables interact with one another and affect the response [Fig. 5]. This helps us to predict the change in response with the change in experimental conditions.
FIGURE 5.
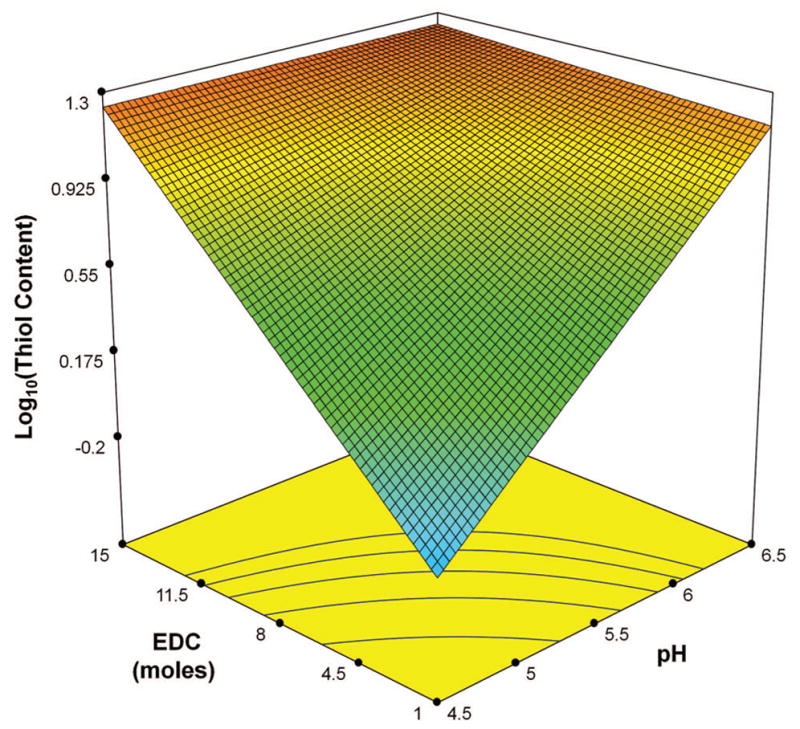
Model graph depicting the nature of interactions between the factors EDC and the pH of the reaction medium in response to amidation at constant values of other factors of Cys = 10 (moles), NHS = 0.1 (moles), time = 3.5 h for FF design.
 represents the gradient of colors with increasing Log10 (Thiol Content) from −0.57 to 1.47. Note that thiol content is a surrogate for amide content.
represents the gradient of colors with increasing Log10 (Thiol Content) from −0.57 to 1.47. Note that thiol content is a surrogate for amide content.
D-optimal design of RSM
Response-surface methodology is used to evaluate the nonlinear effects of factors and search for optimum conditions to stabilize the response. This methodology is useful for quantifying the relationships between the factors and one or more responses to determine optimal conditions. Of several design in RSM, D-Optimal design maximizes the determinant of the information matrix.28,29 We used this design to investigate the factors affecting the response and to build empirical models for optimizing their formulations. Two significant factors considered for the analysis were EDC and Cys [Table III]. The FF results also show pH as a significant single factor affecting the response. A pH of 6.5 is considered for RSM since, statistically, pH in the range of 4.5 to 6.5 had a positive effect on the response. Also, at pH lower than 6.5, the carboxylate moieties of HA are less dissociated, which makes HA less reactive to attack the electron-deficient carbon atom of EDC. At pH higher than 6.5, hydrolysis of the intermediate may occur. The experimental results required a log transformation, indicated by the Box-Cox plot of power transforms. The results were fitted into a second-order response surface model, a quadratic fit, for which the polynomial equation is as follows:
| (3) |
where 0.69 is the intercept of the data.
Analysis of RSM
The ANOVA for the results of disulfide tests indicated that the quadratic regression models were statistically significant, with an R2 of 0.91 and no significant lack of fit [Table IV]. The R2 value indicates a good correlation between the experimental and predicted model. The adjusted R2 that tests the goodness-of-fit of the regression equation was 0.88. Also, the model had a high F-value, 39.43, from the F-test and a p value of < 0.001. This quadratic model was graphically represented by the three dimensional response surfaces. We observed the effect of EDC and Cys, and their mutual interaction over the response from the graphs [Fig. 6]. Also, we noted a normal distribution of residuals with the theoretical values from the normal probability plot of residuals. We found that there are no outliers to be investigated and that the run was consistent with the rest of the data for this model using the externally studentized residual plot.
FIGURE 6.
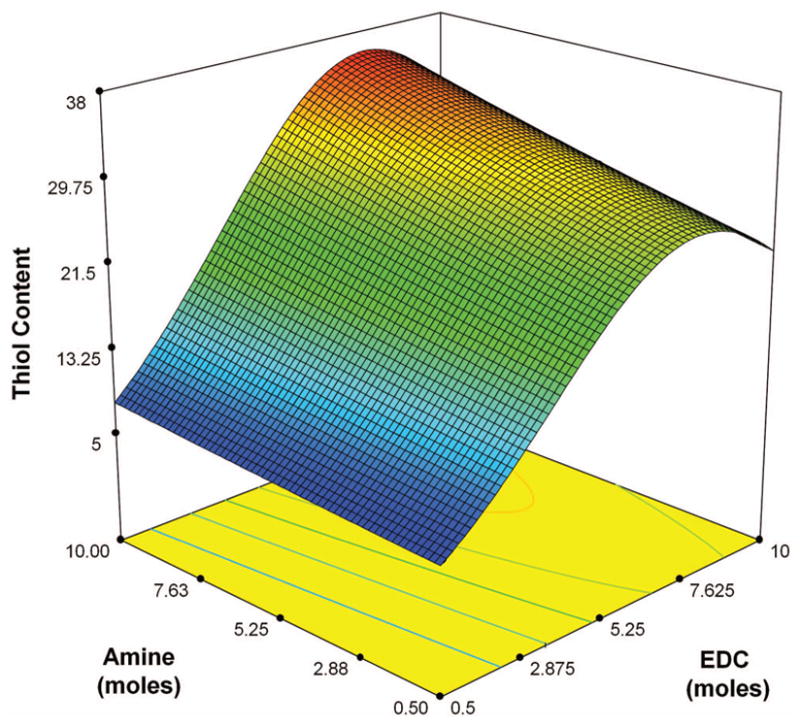
Model graph depicting the nature of interaction between factors EDC and Cys in response to thiol derivatization at constant values of other factors of pH = 6.5, NHS = 0.5 (moles), and time = 3.5 h D-optimal design of RSM.
 represents the gradient of colors with increasing thiol content from 5.52 to 37.12
represents the gradient of colors with increasing thiol content from 5.52 to 37.12
From the ANOVA and the residual analysis, we observed that the significant factor controlling the amidation reaction is EDC rather than amine. As shown by analysis of the reaction mechanism [Fig. 1], HA reacts with EDC to give the intermediate, O-acylisourea. The intermediate reacts with NHS to give a stable NHS-ester, followed by its reaction with cystamine yielding the desired product. However, this may be accompanied by certain side reactions. One of the drawbacks of the carbodiimide-based condensation is that the intermediate O-acylisourea can react not only with primary amine and NHS, but also with EDC and water.30–32 The side reaction of the intermediate O-acylisourea with water leads to hydrolysis and decreases the efficacy of EDC. Hydrolysis of the intermediate O-acylisourea produces urea as the side product which can be removed by dialysis. The side reaction of the intermediate O-acylisourea with EDC produces the side product N-acylurea on the carboxyl groups, especially when excess amount of EDC is used during the reaction. In this study, the carboxyl groups are on the backbone of HA, which makes the removal of the side product N-acylurea very difficult. We measured the contents of N-acylurea by 1H NMR in D2O [Fig. 7]. The integration from 3.1 to 3.9 ppm, which corresponds to the methine groups on the six-membered rings, was set as 10, and was used as a standard to calculate other contents.
FIGURE 7.
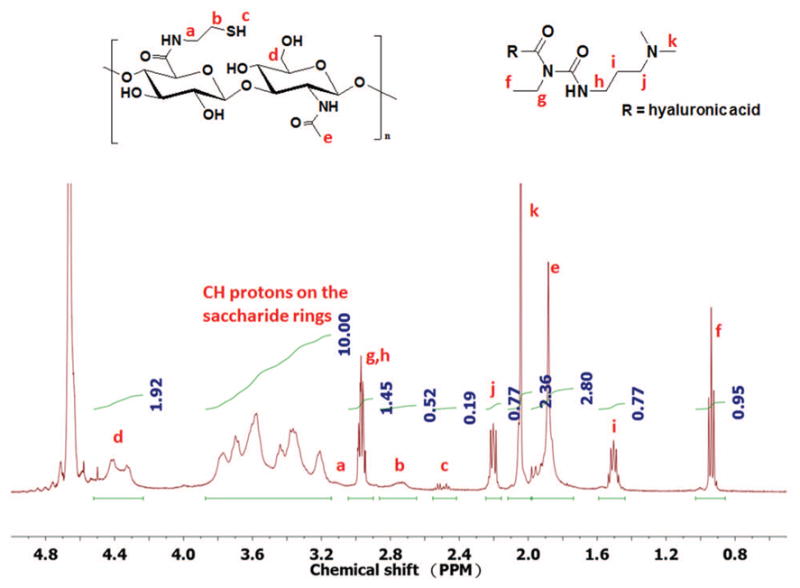
A characteristic 1H NMR spectrum of amidated HA with side product
Statistically analyzing the correlation between this side reaction and the reactant formulation, the significant factors controlling the reaction are EDC and (EDC)2. It follows a second order quadratic fit with a square root transformation given by the polynomial equation:
| (4) |
It is evident from ANOVA that this quadratic regression model is statistically significant with p value < 0.0001 and model F value of 56.09. The lack of fit F-value is 0.77 which implies it is not significant and the quadratic fit is apt for this model [Table IV]. The graphical correlation of the side reaction with factors Amine and EDC is shown in [Fig. 8]. The statistical analysis also confirms that this side reaction is mainly caused by EDC, which is consistent with the mechanism mentioned above. Another possible reaction between HA and cystamine, apart from the side reactions of intermediate, is that the terminal aldehyde group of polysaccharide in its open-chain form can react with a primary amine to form a Schiff-base.33–35 This introduces a terminal thiol group to the HA. However, the amount of terminal aldehyde group in open-chain form is relatively small compared to the side NHS-activated carboxyl groups.
FIGURE 8.
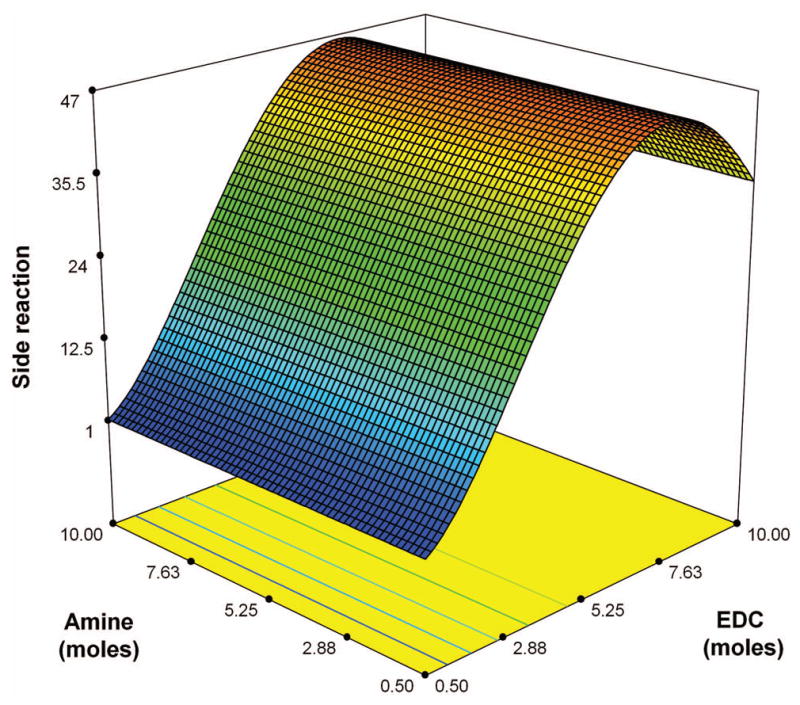
Model graph depicting the nature of interaction between factors EDC and Cys in response to the side reaction.
 represents the gradient of colors with increasing thiol content from 0 to 51.
represents the gradient of colors with increasing thiol content from 0 to 51.
In summary, EDC is the critical factor which controls the desired as well as the undesired substitution. However, since the concentration of NHS was kept constant for RSM design in this study, the effect of NHS on the side reaction is not investigated. But it is noteworthy that, using sufficient amount of NHS would be critical to suppress the formation of the side product N-acylurea because NHS competes with EDC to react with the intermediate O-acylisourea.
Optimization of factors
The aim of this study was to optimize the amidation reaction to determine the experimental conditions and the formulations needed to synthesize HA hydrogels of a specified degree of amidation and thus thiolation. DoE predicts the optimal formulations for carrying out the amidation reaction with the help of the polynomial model equation [Eq. 3] for any specified degree of amidation. For instance, to amidate 7.5 mole % of HA (based on repeat units) with minimal side reactions, the optimal formulation would be 0.5 moles of EDC and NHS (each) and 8 moles of Cys with the pH of the reaction medium at 6.5 for 3.5 h at 37°C. This formulation is effective in producing the desired amidation of HA with negligible side product.
CONCLUSION
The chemical modification of HA plays a significant role in designing the scaffolds for its use in applications such as tissue engineering, targeted drug delivery via thiol-modified-HA coated gold nanoparticles, and permanent vitreous substitute with in-vivo gelling properties. Pre-determining the degree of derivatization of HA is critical for defining the mechanical properties of the scaffolds specific to its application. We investigated the factors controlling derivatization of HA through the carbodiimide reaction and determined the quadratic polynomial model to obtain hydrogels with desired degree of amidation. We designed the experiments and analyzed the carbodiimide reaction with the help of DoE statistics involving FF and RSM. The percentage derivatization of HA via this mechanism is dependent on the formulation of reactants and experimental conditions. Based on the formulation of the reactants, the yield of desired product and the side product can be effectively controlled. EDC is the critical factor which controls the desired as well as the undesired substitution. An adequate quadratic polynomial model for predicting the degree of thiolation and the side reaction from the response surface design is respectively:
This model can be used to design optimal formulations of the reaction to obtain the desirable degree of amidation with cystamine on HA with minimal side reaction.
Acknowledgments
This research was supported by a Department of Veterans Affairs rehab merit review grant RX000657-01 and an NIH grant, EY021620 to Dr. Nathan Ravi. Also, Research to Prevent Blindness, Inc., and NIH Core Grant P30 EY02687 provided support. We acknowledge the use of Design-Expert® software, version 7, Stat-Ease, Inc., Minneapolis, MN, USA, www.statease.com, for our statistical analysis.
References
- 1.Volpi N, Schiller J, Stern R, Soltes L. Role, Metabolism, Chemical Modifications and Applications of Hyaluronan. Current Medicinal Chemistry. 2009;16(14):1718–1745. doi: 10.2174/092986709788186138. [DOI] [PubMed] [Google Scholar]
- 2.Allison DD, Grande-Allen KJ. Review Hyaluronan: A powerful tissue engineering tool. Tissue Engineering. 2006;12(8):2131–2140. doi: 10.1089/ten.2006.12.2131. [DOI] [PubMed] [Google Scholar]
- 3.Nakagawa M, Tanaka M, Miyata T. Evaluation of collagen gel and hyaluronic acid as vitreous substitutes. Ophthalmic Research. 1997;29(6):409–420. doi: 10.1159/000268042. [DOI] [PubMed] [Google Scholar]
- 4.Elia R, Fuegy PW, VanDelden A, Firpo MA, Prestwich GD, Peattie RA. Stimulation of in vivo angiogenesis by in situ crosslinked, dual growth factor-loaded, glycosaminoglycan hydrogels. Biomaterials. 2010;31(17):4630–8. doi: 10.1016/j.biomaterials.2010.02.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Choh SY, Cross D, Wang C. Facile synthesis and characterization of disulfide-cross-linked hyaluronic acid hydrogels for protein delivery and cell encapsulation. Biomacromolecules. 2011;12(4):1126–36. doi: 10.1021/bm101451k. [DOI] [PubMed] [Google Scholar]
- 6.Burdick JA, Prestwich GD. Hyaluronic acid hydrogels for biomedical applications. Adv Mater. 2011;23(12):H41–56. doi: 10.1002/adma.201003963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Eng D, Caplan M, Preul M, Panitch A. Hyaluronan scaffolds: A balance between backbone functionalization and bioactivity. Acta Biomaterialia. 2010;6(7):2407–2414. doi: 10.1016/j.actbio.2009.12.049. [DOI] [PubMed] [Google Scholar]
- 8.Oh EJ, Park K, Kim KS, Kim J, Yang J-A, Kong J-H, Lee MY, Hoffman AS, Hahn SK. Target specific and long-acting delivery of protein, peptide, and nucleotide therapeutics using hyaluronic acid derivatives. Journal of Controlled Release. 2010;141(1):2–12. doi: 10.1016/j.jconrel.2009.09.010. [DOI] [PubMed] [Google Scholar]
- 9.Lacerda SH, Park JJ, Meuse C, Pristinski D, Becker ML, Karim A, Douglas JF. Interaction of gold nanoparticles with common human blood proteins. ACS Nano. 2010;4(1):365–79. doi: 10.1021/nn9011187. [DOI] [PubMed] [Google Scholar]
- 10.Papasani MR, Wang G, Hill RA. Gold nanoparticles: the importance of physiological principles to devise strategies for targeted drug delivery. Nanomedicine. 2012;8(6):804–814. doi: 10.1016/j.nano.2012.01.008. [DOI] [PubMed] [Google Scholar]
- 11.Kafedjiiski K, Jetti RK, Foger F, Hoyer H, Werle M, Hoffer M, Bernkop-Schnurch A. Synthesis and in vitro evaluation of thiolated hyaluronic acid for mucoadhesive drug delivery. Int J Pharm. 2007;343(1–2):48–58. doi: 10.1016/j.ijpharm.2007.04.019. [DOI] [PubMed] [Google Scholar]
- 12.Lu PL, Lai JY, Ma DH, Hsiue GH. Carbodiimide cross-linked hyaluronic acid hydrogels as cell sheet delivery vehicles: characterization and interaction with corneal endothelial cells. J Biomater Sci Polym Ed. 2008;19(1):1–18. doi: 10.1163/156856208783227695. [DOI] [PubMed] [Google Scholar]
- 13.Kuo JW, Swann DA, Prestwich GD. Chemical modification of hyaluronic acid by carbodiimides. Bioconjug Chem. 1991;2(4):232–41. doi: 10.1021/bc00010a007. [DOI] [PubMed] [Google Scholar]
- 14.Peng HT, Blostein MD, Shek PN. Experimental Optimization of an In Situ Forming Hydrogel for Hemorrhage Control. Journal of Biomedical Materials Research Part B-Applied Biomaterials. 2009;89B(1):199–209. doi: 10.1002/jbm.b.31206. [DOI] [PubMed] [Google Scholar]
- 15.Narayan PM, Marchant D, Wheatley MA. Optimization of spray drying by factorial design for production of hollow microspheres for ultrasound imaging. Journal of Biomedical Materials Research. 2001;56(3):333–341. doi: 10.1002/1097-4636(20010905)56:3<333::aid-jbm1101>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- 16.Ma Y, Zhang Y, Zhao S, Wang Y, Wang S, Zhou Y, Li N, Xie H, Yu W, Liu Y, et al. Modeling and optimization of membrane preparation conditions of the alginate-based microcapsules with response surface methodology. J Biomed Mater Res A. 2012;100(4):989–98. doi: 10.1002/jbm.a.34032. [DOI] [PubMed] [Google Scholar]
- 17.Abul Kalam M, Sultana Y, Ali A, Aqil M, Mishra AK, Aljuffali IA, Alshamsan A. Part I: Development and optimization of solid-lipid nanoparticles using Box-Behnken statistical design for ocular delivery of gatifloxacin. Journal of Biomedical Materials Research Part A. 2013;101(6):1813–1827. doi: 10.1002/jbm.a.34453. [DOI] [PubMed] [Google Scholar]
- 18.Nottelet B, Pektok E, Mandracchia D, Tille JC, Walpoth B, Gurny R, Moller M. Factorial design optimization and in vivo feasibility of poly(epsilon-caprolactone)-micro- and narofiber-based small diameter vascular grafts. Journal of Biomedical Materials Research Part A. 2009;89A(4):865–875. doi: 10.1002/jbm.a.32023. [DOI] [PubMed] [Google Scholar]
- 19.Thannhauser TW, Konishi Y, Scheraga HA. Analysis for disulfide bonds in peptides and proteins. Methods Enzymol. 1987;143:115–9. doi: 10.1016/0076-6879(87)43020-6. [DOI] [PubMed] [Google Scholar]
- 20.Ellman GL. Tissue sulfhydryl groups. Archives of Biochemistry and Biophysics. 1959;82(1):70–77. doi: 10.1016/0003-9861(59)90090-6. [DOI] [PubMed] [Google Scholar]
- 21.Park SN, Park JC, Kim HO, Song MJ, Suh H. Characterization of porous collagen/hyaluronic acid scaffold modified by 1-ethyl-3-(3-dimethylaminopropyl)carbodiimide cross-linking. Biomaterials. 2002;23(4):1205–12. doi: 10.1016/s0142-9612(01)00235-6. [DOI] [PubMed] [Google Scholar]
- 22.Sehgal D, Vijay IK. A method for the high efficiency of water-soluble carbodiimide-mediated amidation. Anal Biochem. 1994;218(1):87–91. doi: 10.1006/abio.1994.1144. [DOI] [PubMed] [Google Scholar]
- 23.Cao H, Xu SY. EDC/NHS-crosslinked type II collagen-chondroitin sulfate scaffold: characterization and in vitro evaluation. J Mater Sci Mater Med. 2008;19(2):567–75. doi: 10.1007/s10856-007-3281-5. [DOI] [PubMed] [Google Scholar]
- 24.Davidenko N, Campbell JJ, Thian ES, Watson CJ, Cameron RE. Collagen-hyaluronic acid scaffolds for adipose tissue engineering. Acta Biomater. 2010;6(10):3957–68. doi: 10.1016/j.actbio.2010.05.005. [DOI] [PubMed] [Google Scholar]
- 25.Wang TW, Spector M. Development of hyaluronic acid-based scaffolds for brain tissue engineering. Acta Biomater. 2009;5(7):2371–84. doi: 10.1016/j.actbio.2009.03.033. [DOI] [PubMed] [Google Scholar]
- 26.Everaerts F, Torrianni M, Hendriks M, Feijen J. Quantification of carboxyl groups in carbodiimide cross-linked collagen sponges. J Biomed Mater Res A. 2007;83(4):1176–83. doi: 10.1002/jbm.a.31398. [DOI] [PubMed] [Google Scholar]
- 27.Taguchi T, Ikoma T, Tanaka J. An improved method to prepare hyaluronic acid and type II collagen composite matrices. J Biomed Mater Res. 2002;61(2):330–6. doi: 10.1002/jbm.10147. [DOI] [PubMed] [Google Scholar]
- 28.Valencia P, Cornejo I, Almonacid S, Teixeira AA, Simpson R. Kinetic Parameter Determination for Enzyme Hydrolysis of Fish Protein Residue Using D-optimal Design. Food and Bioprocess Technology. 2013;6(1):290–296. [Google Scholar]
- 29.Motealleh B, Zahedi P, Rezaeian I, Moghimi M, Abdolghaffari AH, Zarandi MA. Morphology, drug release, antibacterial, cell proliferation, and histology studies of chamomile-loaded wound dressing mats based on electrospun nanofibrous poly(epsilon-caprolactone)/polystyrene blends. Journal of Biomedical Materials Research Part B-Applied Biomaterials. 2014;102(5):977–987. doi: 10.1002/jbm.b.33078. [DOI] [PubMed] [Google Scholar]
- 30.Nakajima N, Ikada Y. Mechanism of Amide Formation by Carbodiimide for Bioconjugation in Aqueous-Media. Bioconjugate Chemistry. 1995;6(1):123–130. doi: 10.1021/bc00031a015. [DOI] [PubMed] [Google Scholar]
- 31.Palazon F, Benavides CM, Leonard D, Souteyrand E, Chevolot Y, Cloarec JP. Carbodiimide/NHS Derivatization of COOH-Terminated SAMs: Activation or Byproduct Formation? Langmuir. 2014;30(16):4545–4550. doi: 10.1021/la5004269. [DOI] [PubMed] [Google Scholar]
- 32.Bulpitt P, Aeschlimann D. New strategy for chemical modification of hyaluronic acid: Preparation of functionalized derivatives and their use in the formation of novel biocompatible hydrogels. Journal of Biomedical Materials Research. 1999;47(2):152–169. doi: 10.1002/(sici)1097-4636(199911)47:2<152::aid-jbm5>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 33.Lee MY, Yang JA, Jung HS, Beack S, Choi JE, Hur W, Koo H, Kim K, Yoon SK, Hahn SK. Hyaluronic Acid-Gold Nanoparticle/Interferon alpha Complex for Targeted Treatment of Hepatitis C Virus Infection. Acs Nano. 2012;6(11):9522–9531. doi: 10.1021/nn302538y. [DOI] [PubMed] [Google Scholar]
- 34.Lee H, Lee MY, Bhang SH, Kim BS, Kim YS, Ju JH, Kim KS, Hahn SK. Hyaluronate-Gold Nanoparticle/Tocilizumab Complex for the Treatment of Rheumatoid Arthritis. Acs Nano. 2014;8(5):4790–4798. doi: 10.1021/nn500685h. [DOI] [PubMed] [Google Scholar]
- 35.Kim WJ, Sato Y, Akaike T, Maruyama A. Cationic comb-type copolymers for DNA analysis. Nature Materials. 2003;2(12):815–820. doi: 10.1038/nmat1021. [DOI] [PubMed] [Google Scholar]