Abstract
Objectives
Determine if direct tumor cell cytotoxicity, antigen release, and susceptibility to T-lymphocyte killing following radiation treatment is dose-dependent.
Materials and Methods
Mouse oral cancer cells were engineered to express full-length ovalbumin as a model antigen. Tumor antigen release with uptake and cross presentation of antigen by antigen presenting cells with subsequent priming and expansion of antigen-specific T-lymphocytes following radiation was modeled in vitro and in vivo. T-lymphocyte mediated killing was measured following radiation treatment using a novel impedance-based cytotoxicity assay.
Results
Radiation treatment induced dose-dependent induction of executioner caspase activity and apoptosis in MOC1 cells. In vitro modeling of antigen release and T-lymphocyte priming demonstrated enhanced proliferation of OT-1 T-lymphocytes with 8 Gy treatment of MOC1ova cells compared to 2 Gy. This was validated in vivo following treatment of established MOC1ova tumors and adoptive transfer of antigen-specific T-lymphocytes. Using a novel impedance – based cytotoxicity assay, 8 Gy enhanced tumor cell susceptibility to T-lymphocyte killing to a greater degree than 2 Gy.
Conclusion
In the context of using clinically-relevant doses of radiation treatment as an adjuvant for immunotherapy, 8 Gy is superior to 2 Gy for induction of antigen-specific immune responses and enhancing tumor cell susceptibility to T-lymphocyte killing. These findings have significant implications for the design of trials combining radiation and immunotherapy.
Keywords: Radiation, immunity, tumor microenvironment, T-lymphocyte priming, cytotoxic T-lymphocyte
Introduction
While many patients with head and neck squamous cell carcinoma (HNSCC) display a T-cell inflamed phenotype[1], only a small subset respond to programmed death (PD) pathway checkpoint inhibition[2]. Strategies to enhance response rates to checkpoint inhibition in HNSCC are needed. PD-based checkpoint blockade has the potential to unleash an existing anti-tumor immune response being blocked by the expression of PD-1/PD-L1, but cannot induce a de novo anti-tumor immunity[3]. The addition of PD-based checkpoint blockade to other anti-cancer treatments that have the potential to induce adaptive anti-tumor immune responses may be additive or synergistic due to reversal of adaptive immune resistance[3, 4]. Ionizing radiation (IR) is a mainstay of treatment for HNSCC and can induce anti-tumor immune responses via a number of defined mechanisms[5–7].
To provide a rationale for combining IR with immune-activating treatments, we hypothesized that IR could induce tumor cell death, causing release of tumor antigen for uptake and cross-presentation by antigen presenting cells (APC) with subsequent activation of antigen-specific T-lymphocytes. To accomplish this, we engineered mouse oral cancer (MOC) cells to express full-length ovalbumin as a well-defined model antigen and treated cells or tumors with clinically relevant doses of 2 Gy or 8 Gy IR. We demonstrated dose-dependent antigen release, processing and antigen-specific T-lymphocyte activation both in vitro and in vivo following IR, to a greater degree with 8 Gy than 2 Gy. Similarly, IR also significantly enhanced antigen-specific cytotoxic T-lymphocyte (CTL) killing of target cells in a novel, impedance based cytotoxicity assay to a greater degree with 8 Gy than 2 Gy. Given that standard-of-care treatment for HNSCC involves the use of fractionated low dose (2 Gy) IR, these results suggest that careful consideration should be given to experimental design in the setting of IR being used as an adjuvant treatment with immune-activating therapies such as checkpoint inhibition.
Materials and Methods
Cell culture and tumor growth
Syngeneic mouse oral cancer 1 (MOC1) cells were generated as described[8], cultured as described[9] and harvested with TrypLE Select to avoid cell surface epitope loss. To generate tumors, 5×106 cells were injected subcutaneously into the right leg of wild-type C57BL/6 (B6) mice in 30% matrigel (Corning). All studies involving tumor implantation and irradiation of mice received National Institutes of Health Animal Care and Use Committee approval (ASP#1364-14).
Generation of MOC1ova
A pBABE vector backbone containing full length ovalbumin and resistance genes (ampicillin and puromycin) was kindly provided by Dr. Gavin Dunn (Washington University in St. Louis). This plasmid and the retroviral envelop plasmid VSV-G were transformed into MAX efficiency DH5α cells on ampicillin impregnated LB plates for expansion. Isolation of plasmids was performed using an EndoFree Plasmid Maxi Kit (Qiagen). The ovalbumin and VSV-G plasmids were transfected into 293gp packaging cells in OptiMEM using Lipofectamine 2000. Viral-containing supernatants were collected at 48 hours. To prepare for transduction, MOC1 cells were plated on retronectin (TaKaRa) coated plates pre-seeded with retrovirus via centrifugation of viral supernatant. Following an overnight infection, transduced MOC1 cells were trypsinized and cultured in puromycin at a concentration pre-determined to be lethal to MOC1 cells (6 µg/mL). Transduction of ovalbumin containing plasmid was verified by puromycin resistance, flow cytometry for SIINFEKL presentation on H2-Kb, and cytotoxicity upon exposure to ex vivo generated OT-1 CTLs.
Radiation
Cells were harvested while in log growth phase and irradiated (2 or 8Gy) using a 137Cs source (Gammacell-1000) at a dose rate of 0.74 Gy/min. Irradiated cells were washed three times before being plated for experiments. Mice bearing tumors were secured into custom lead-shielded jigs that expose the leg alone to radiation, and irradiated (2 or 8Gy) using a Pentak XRAD320 X-ray irradiator (Precision X-ray, Inc.) at a dose of 2.8 Gy/min.
Caspase 3/7 and annexin V assay
MOC1 cells were irradiated and cultured for 12 hours before addition of CellEvent Caspase-3/7 Green Detection Reagent (ThermoFisher) per manufacturer protocol. Images were acquired on an Evos Cell Imaging System (ThermoFisher) and % positive cells was calculated manually from 10 high power fields (HPFs) per treatment condition. MOC1 cells were cultured for 24 hours before detection of apoptosis using the flow cytometry-based PE Annexin V Apoptosis Detection Kit I (BD Biosciences) per manufacturer protocol.
Flow cytometry
All analyses were performed on fresh cells or prepared tissue with exclusion of dead cells via 7AAD staining. Anti-SIINFEKL:H2-Kb (clone 25-D1.16), CD45.2 (104), CD11b (M1/70), CD11c (N418), CD19 (6D5), Vα2 (B20.1), ICAM (YN1/1.7.4), CD80 (16-10A1), and Fas (SA367H8) antibodies were from Biolegend and anti-calreticulin antibody (ab92516) was from Abcam. Isotype control antibodies and a “fluorescence minus one” method of antibody combination were used for specific staining validation. Data was acquired on a FACSCanto using FACSDiva software (BD Biosciences) and analyzed on FlowJo software vX10.0.7r2.
Colony formation assay
Clonogenic assays were performed on control or irradiated MOC1ova cells as described[10].
Cytotoxic T-lymphocyte (CTL) killing assay
To generate SIINFEKL-specific CTLs, splenocytes from OT-1 mice were cultured in the presence of SIINFEKL (2 µg/ml) with daily 2:1 splitting. After 72 hours in culture, >80% of remaining cells are CD8+Vα2+ cells (data not shown). Following the addition of OT-1 CTL effectors to 1×104 target cells plated in a 96-well E-Plate (ACEA Biosciences), alteration of impedance was acquired using the xCELLigence Real-Time Cell Analysis (RTCA) platform per manufacturer recommendations. Triton X-100 (0.2%) was added to some wells to verify complete loss of cell index with total cell lysis, and CTLs alone were plated up to 1×106/well to verify that they do not contribute to gain of impedance (data not shown). Percent loss of cell index for a given time point was calculated as: 1-(experimental cell index/control cell index).
In vitro cross-presentation and T-lymphocyte priming assay
MOC1ova cells (3×105) were irradiated and immediately placed in co-culture with unsorted wild-type B6 splenocytes at a 1:1 ratio with or without recombinant murine IFNβ at 500 U/mL. After 48 hours of co-culture, cells were harvested and either analyzed for SIINFEKL epitope cross-presentation of H2-Kb via flow cytometry using cell lineage markers or used as stimulators for naïve, sorted, CFSE-labelled OT-1 T-lymphocytes. Sorting of T-lymphocytes from whole spleen was performed using negative magnetic separation strategies (AutoMACS, Miltenyi) and the Pan T-cell Isolation Kit II (Miltenyi). T-lymphocytes were labelled with 5 µM CFSE (Sigma) for 3 minutes before quenching with 1×PBS/5% heat-inactivated FBS. Proliferation of CFSE-labelled T-lymphocytes was analyzed via flow cytometry and the division index was calculated via FlowJo software as described[11].
In vivo T-lymphocyte priming assay
Mice bearing MOC1ova tumors were irradiated and immediately tail-vein injected with 5–8×106 CFSE-labelled OT-1 T-lymphocytes. After 72 hours, spleens, tumor-draining lymph nodes and tumors were harvested and prepared as described[12]. Single cell suspensions were stained with primary conjugated antibodies and analyzed for CFSE-distribution of 7AAD−CD45.2+Vα2+ OT-1 T-lymphocytes by flow cytometry with calculation of division index as described above.
Statistical analysis
Tests of significance between pairs of data are reported as p-values, derived using a student’s t-test with a two-tailed distribution and calculated at 95% confidence. Comparison of multiple sets of data was achieved with analysis of variance (ANOVA) with Tukey’s multiple comparisons. All error bars indicate standard deviation. Statistical significance was set to p<0.05. All analysis was performed using GraphPad Prism v7.
Results
Higher doses of radiation induce greater executioner caspase activation and apoptosis
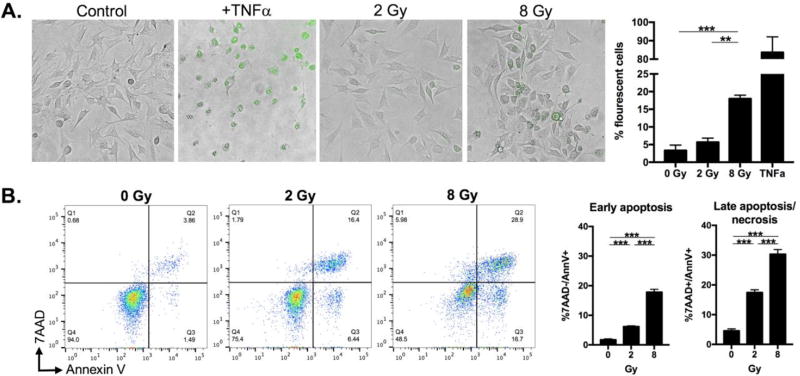
IR induces caspase-3-dependent apoptosis[13]. To determine if there was dose-dependent induction of caspase-3 activity, we exposed MOC1 cells to a single fraction of 0, 2 or 8 Gy and measured caspase-3/7 activity using a fluorescent DEVD peptide/DNA dye conjugate. Within 12 hours of exposure to IR, MOC1 cells treated with 8 Gy demonstrated significantly more caspase-3/7 activity than cells treated with 2 Gy (Figure 1A, TNF as a positive control). To determine if this increase correlated with increased apoptosis, cells were stained with annexin V and 7AAD 24 hours after exposure to IR. Treatment with 8 Gy induced both early and late apoptosis to a greater degree than 2 Gy (Figure 1B). Thus, 8 Gy IR induces executioner caspase activity and an apoptotic phenotype to a greater degree than 2 Gy in MOC1 cells.
Figure 1. Ionizing radiation dose-dependent induction of caspase 3/7 activity and apoptosis.
A, induction of caspase 3/7 activity after a single dose of ionizing radiation. MOC1 cells were irradiated and cultured for 12 hours before caspase 3/7 detection reagent added. Representative photomicrographs at 20× magnification. TNFα (100 ng/mL) used as positive control. Quantification from 10 HPFs per condition. B, induction of apoptosis and necrosis after a single dose of ionizing radiation. MOC1 cells were irradiated, cultured for 24 hours, harvested, and immediately stained and analyzed via flow cytometry. Late apoptosis/necrosis defined as annexin V and 7AAD double positive; early apoptosis as annexin V positive but 7AAD negative. Representative results from one of at least three independent experiments are shown. **, p,0.01; ***, p<0.001.
Generation of MOC1 cells expressing a well-defined model antigen
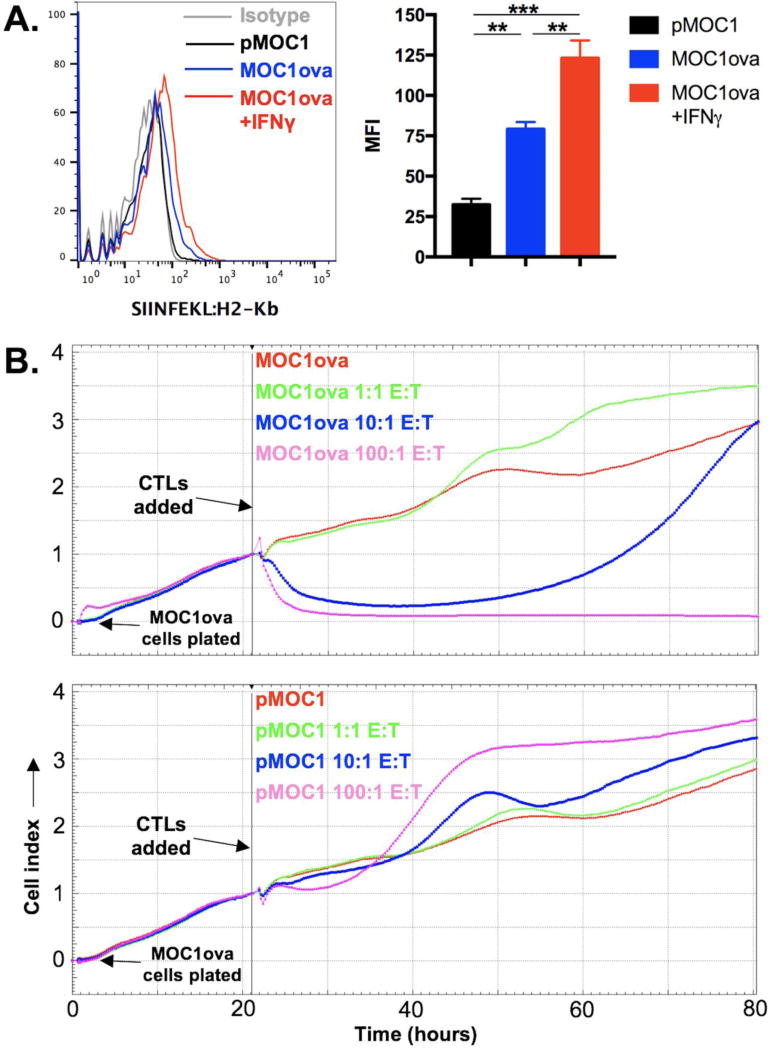
To study antigen release from dead or dying tumor cells, we engineered MOC1 cells to express a well-defined antigen. MOC1 cells were stably transduced with a plasmid encoding full length ovalbumin as a model antigen. To measure cell surface presentation of the known ovalbumin MHC class I epitope SIINFEKL, we stained cells with an antibody that specifically binds SIINFEKL presented on MHC class I H2-Kb. Flow cytometric analysis reveals IFNγ-inducible presentation of SIINFEKL on the surface of MOC1ova cells (Figure 2A). Relatively low SIINFEKL presentation is achieved even in the presence of IFNγ, possibly due to competition within the cell for peptide processing and MHC class I loading. To validate that SINFEKL presented on the surface of MOC1ova cells serves as a CTL antigen, we performed CTL assays using ex vivo generated OT-1 CTLs as effectors in an impedance-based cytotoxicity assay. OT-1 CTLs induced E:T ratio-dependent loss of MOC1ova (Figure 2B, top panel) but not parental MOC1 cell index (Figure 2B, bottom panel). As a measure of cytotoxicity, a 100:1 E:T ratio induces complete loss of MOC1ova cell index, whereas 1:1 E:T ratio induces no loss of cell index. These data validate the expression and processing of full length ovalbumin into the MHC class restricted peptide SIINFEKL that serves as a CTL antigen on the surface of MOC1ova cells.
Figure 2. Validation of MOC1 cells engineered to express full-length ovalbumin as a model antigen.
A, MOC1 cells stably transduced with full length ovalbumin were exposed to IFNγ (10 ng/mL) for 24 hours and assessed via flow cytometry for presentation of SIINFEKL on H2-Kb. Histograms displayed on the left, MFI quantification on the right. B, using changes in impedance as a measure of target cell killing, ex vivo generated OT-1 CTLs were applied to either MOC1ova (top panel) or parental MOC1 (bottom panel) cells at the indicated E:T ratios. Target cells were plated and impedance was allowed to rise for 22 hours before the addition of effectors. Representative results from one of at least three independent experiments are shown. **, p,0.01; ***, p<0.001.
Higher doses of radiation induce antigen release, cross presentation and priming of antigen-specific T-lymphocytes in vitro
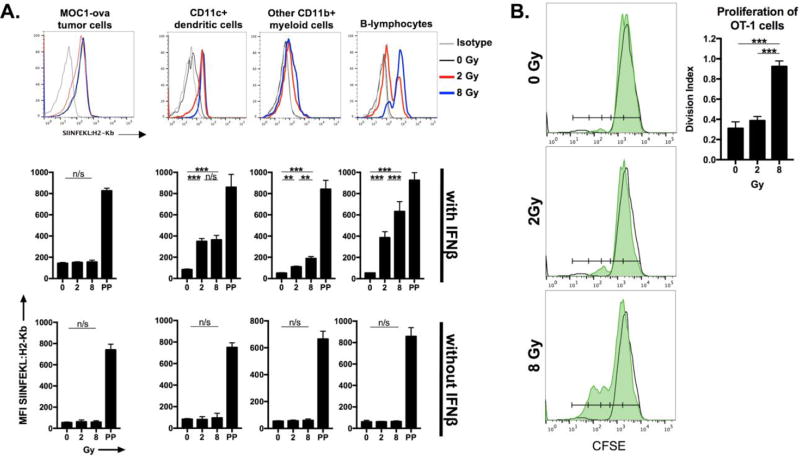
To model whether IR can induce antigen release and subsequent priming of T-lymphocytes in vitro, we first assessed whether splenocytes serving as antigen presenting cells (APCs) could take up ovalbumin released by irradiated cells and cross-present SIINFEKL on H2-Kb. Figure 3A demonstrates representative histograms and quantification of SIINFEKL presentation via H2-Kb on the surface of CD11c+ dendritic cells (DCs), other CD11b+ myeloid cells and CD19+ B-lymphocytes. APCs exposed to irradiated MOC1ova cells cross-presented SIINFEKL, to a greater degree with 8 Gy than 2 Gy in myeloid cells and B-lymphocytes and to a similar degree in DCs. Cross-presentation of SIINFEKL on APC appeared to be dependent upon the presence of IFNβ (lower panels), consistent with the work of Fuertes et al. and Diamond et al. [14, 15]. Presentation of SIINFEKL via H2-Kb on the surface of the tumor cells did not appear to be significantly enhanced with IR. To evaluate the ability of SIINFEKL presenting APCs to prime antigen-specific T-lymphocytes, APCs exposed to irradiated MOC1ova cells were co-cultured with naïve, CFSE-labelled OT-1 T-lymphocytes (Figure 3B). OT-1 T-lymphocytes exposed to APCs co-cultured with MOC1ova cells irradiated to 8 Gy proliferated to a significantly greater degree. Taken together, these data indicate that 8 but not 2 Gy IR induces antigen release to a degree that it becomes available to APCs for IFNβ-dependent cross-presentation and activation of antigen-specific T-lymphocytes.
Figure 3. Antigen cross-presentation and T-lymphocyte priming in vitro following ionizing radiation.
A, MOC1ova cells were irradiated and cultured with naïve B6 splenocytes in the presence or absence of IFNβ (500 units/mL). Co-cultured cells were harvested and tumor cells (CD45.2−), dendritic cells (CD11b+/−CD11c+), other myeloid cells (CD11b+CD11c−) and B-lymphocytes (CD19+) were assessed for cell surface SIINFEKL:H2-Kb via flow cytometry. In the bar graph quantifying SIINFEKL presentation, also quantified is SIINFEKL presentation following SIINFEKL peptide pulse (PP; 2 µg/mL × 4 hours) as a representation of antibody specificity and for comparison purposes. B, following co-culture of irradiated MOC1ova cells and naive B6 splenocytes in the presence of IFNβ for 48 hours, splenocytes were co-cultured with sorted, CFSE-labelled naïve OT-1 T-lymphocytes for 72 hours. Proliferation of OT-1 T-lymphocytes was measured by CFSE spread on flow cytometry and quantified via calculation of the division index. Unfilled histograms represent histograms of unstimulated CFSE-labelled naïve OT-1 T-lymphocytes alone. Representative results from one of at least two independent experiments are shown. **, p,0.01; ***, p<0.001. n/s, non-significant.
Higher doses of radiation induce priming of antigen-specific T-lymphocytes in vivo
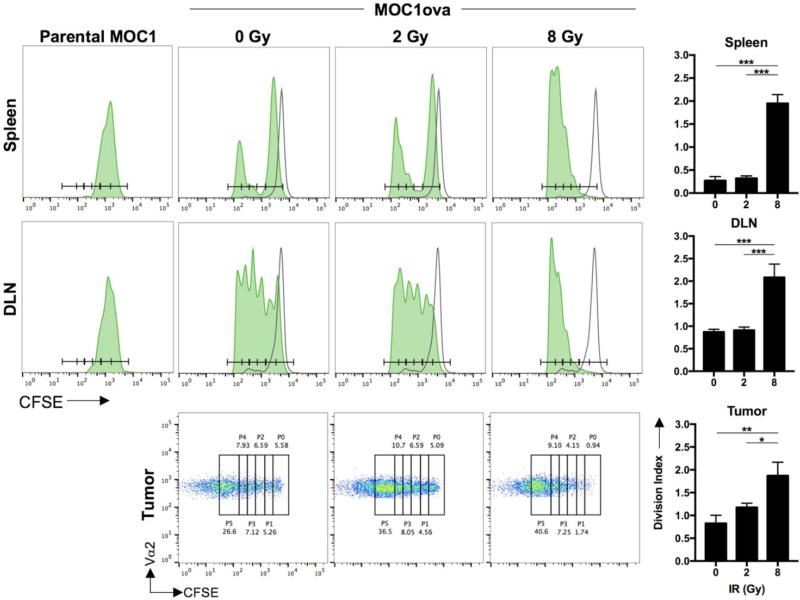
To validate these in vitro findings in vivo, we established MOC1ova tumors in wild-type B6 mice. Following irradiation of tumors with a single dose of 0, 2 or 8 Gy, we assessed the proliferation of adoptively transferred naïve, CFSE labelled OT-1 T-lymphocytes harvested from the spleen, tumor-draining lymph node and tumor. Using mice bearing parental MOC1 tumors without ovalbumin as a negative control, analysis indicated that in vivo baseline OT-1 T-lymphocyte proliferation was enhanced to a greater degree with 8 Gy treatment compared to 2 Gy in all tissue compartments analyzed (Figure 4, representative histograms and dot plots on left and quantification of division index on right). Together, these data indicate enhanced in vivo antigen-specific T-lymphocyte priming and expansion in both the periphery and tumor microenvironment following 8 Gy IR compared to 2 Gy.
Figure 4. Antigen-specific T-lymphocyte priming in vivo following ionizing radiation.
Established (>0.1 cm3 volume) MOC1ova tumors in wild-type B6 mice were irradiated and CFSE-labelled OT-1 T-lymphocytes were adoptively transferred. CFSE spread of Vα2+ T-lymphocytes was assessed from the spleen, tumor-draining lymph node and tumor via flow cytometry (n=5 mice/group). Unfilled histograms represent CFSE-labelled OT-1 T-lymphocytes adoptively transferred into naive, non-tumor bearing mice. Representative CFSE histograms (spleen, lymph node) or dot plots (tumor) are shown with quantification of division index on the right. CFSE spread of adoptively transferred OT-1 T-lymphocytes into parental MOC1 tumor-bearing mice were used as a control for antigen-specific T-lymphocyte proliferation (left histograms). Representative results from one of at least two independent experiments are shown. *, p<0.05; **, p,0.01; ***, p<0.001.
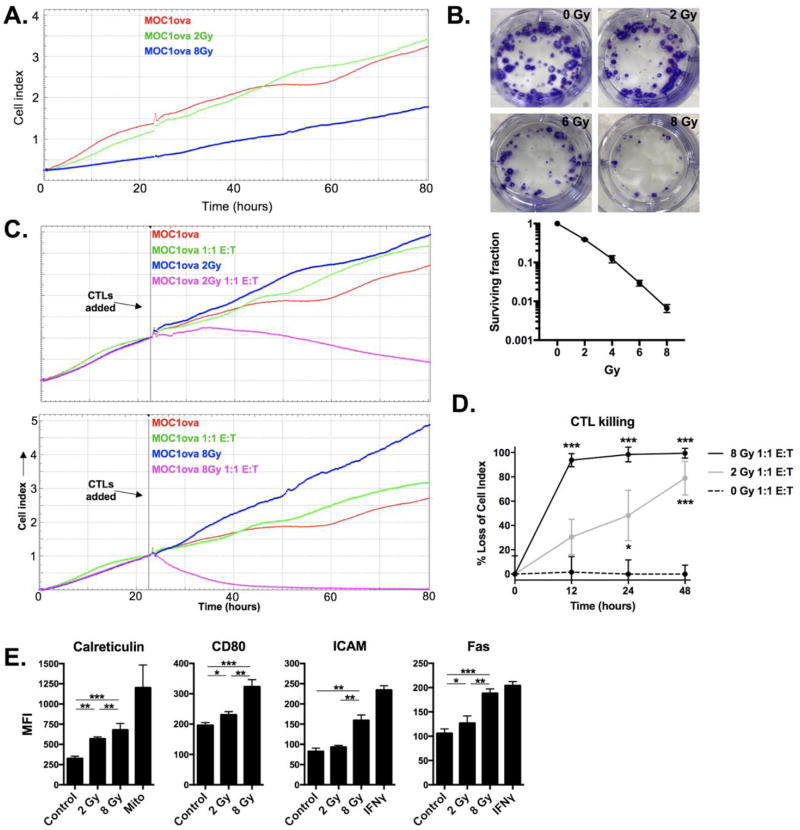
Radiation enhances tumor cell susceptibility to CTL-mediated lysis in a dose-dependent fashion
While IR did not appear to enhance antigen presentation (SIINFEKL:H2-Kb) on the surface MOC1ova cells, we hypothesized that increasing doses of IR would nevertheless sensitize tumor cells to CTL-mediated cytotoxicity. Exposure of MOC1ova cells to 8 Gy IR slowed proliferation compared to 2 Gy treated cells, but did not inhibit outgrowth of cells that survived IR (Figure 5A). To validate these findings and for comparison purposes, colony formation assays revealed that exposure of MOC1ova cells to 8 Gy IR significantly limited MOC1ova survival fraction compared to 2 Gy treated cells (Figure 5B). While OT-1 CTLs at a 1:1 E:T ratio had no measureable effects on non-irradiated MOC1ova cells, IR reversed resistance to CTL-mediated lysis with measureable loss of cell index in MOC1ova cells treated with 2 Gy and near-complete loss of cell index in cells treated with 8 Gy within 12 hours (Figure 5C panels, quantified in 5D). Expression of cell surface calreticulin, ICAM, CD80 and Fas, involved in CTL:target cell interaction and sensitivity to CTL-mediated lysis, was increased in a dose-dependent fashion following IR (Figure 5E). These data support that IR sensitizes MOC1ova cells to CTL-mediated lysis to a greater degree following 8 Gy than 2 Gy, potentially through enhanced CTL:target cell interaction and/or enhanced Fas expression.
Figure 5. Enhanced sensitivity to CTL-mediated killing following ionizing radiation.
A, MOC1ova cells were irradiated, cultured, and growth was measured via change in impedance. Data is not normalized to any time point. B, cells were irradiated with either 2, 4, 6 or 8 Gy, and standard clonogenic assays were performed. Representative photos of wells demonstrating colony formation for each condition are shown on top panels, with survival fraction quantified below. Two independent experiments were performed with similar results. C, irradiated MOC1ova cells were co-cultured with ex vivo generated OT-1 CTLs at a 1:1 E:T ratio. Comparative impedance curves for each experimental condition (2 vs 8 Gy) were normalized to the time at which effectors were added (vertical black line). C, percent loss of cell index quantified at 12, 24 and 48 hours after the addition of effector CTLs. Asterisks indicate significant differences compared to 0 Gy. D, MFI quantification of cell surface expression of immunogenicity markers on MOC1 cells 24 hours following IR or the addition of positive control (mitoxantrone 1 µM; IFNγ 10 ng/mL). Representative results from one of three independent experiments are shown. *, p<0.01; **, p<0.01; ***, p<0.001.
Discussion
With the FDA-approval of immune checkpoint inhibitors, immunotherapy has emerged as an exciting new treatment option for patients with recurrent/metastatic HNSCC. Combining checkpoint inhibition with standard of care therapies, such as IR, may enhance response rates through a number of mechanims. Appropriately executed pre-clinical research is critical to inform the design of clinical trials aiming to demonstrate such enhanced responses. IR is attractive as a potential adjuvant to immunotherapy given its widespread use in the definitive management of both early and advanced stage HNSCC[16] as well as its demonstrated ability to enhance responses to many types of immunotherapy under specific experimental conditions[7, 17–21].
Here we have demonstrated that a single dose of 8 Gy is superior to 2 Gy in terms of both tumor cell antigen release with subsequent priming of antigen specific T–lymphocytes by cross-presenting APCs and direct enhanced tumor cell sensitivity to CTL killing. Our use of well-established oral cavity tumor cells (MOC1) engineered to express full length ovalbumin allowed us to model antigen release, uptake and cross presentation by APCs and stimulation of antigen-specific T-lymphocytes both in vitro and in vivo. Dose-dependent induction of MOC1 apoptosis following IR is significant given evidence that apoptotic cells serve as efficient antigen sources[22]. Our demonstration of the absolute requirement of type I interferon for effective APC antigen cross-presentation supports the work of Diamond et al.[15] and Fuertes et al.[14] demonstrating abrogation of cross presentation and activation of anti-tumor immunity with loss of type I IFN receptor expression. With our MOC1ova model system, it is unclear if the antigen available for uptake by APCs at baseline or following IR is in the form of cellular (tumor cell debris) or free antigen. Others have demonstrated enhanced priming of antigen-specific T-lymphocytes in melanoma (B16) tumor draining lymph nodes following IR using similar techniques[23, 24], but have utilized higher single doses (15–20 Gy) that may not be feasible clinically in patients with HNSCC. This work is the first to demonstrate dose-dependent priming of adoptively transferred antigen-specific T-lymphocytes in vivo following IR in the peripheral compartment, TDLN and tumor microenvironment following IR treatment at doses clinically relevant to HNSCC.
Though cross presentation of cellular antigen is typically a function attributed to CD11c+ DCs, our findings of antigen cross-presentation in vitro by CD11c+ DCs, other CD11c− myeloid cells and B-lymphocytes is supported by the findings of others[25, 26]. We do not know which professional APC cell type is ultimately responsible for cross presentation of antigen in vivo in our model system, but this could be achieved with selective antibody-based elimination or genetic alteration of specific APC types within IR treated mice bearing MOC1ova tumors.
Enhanced CTL lysis of irradiated tumor cells has been demonstrated in a number of cancer models[5, 6, 27]. Traditional CTL assays utilize release of isotopes that allow quantification of target cell lysis at one specific time point, typically 4 hours. Use of the impedance-based CTL assay, which continuously measures the adherence of target cells following the addition of effector CTLs, allows real-time analysis of target cell cytotoxicity kinetics for an extended period with high sensitivity. Here we were able to demonstrate complete loss of cell impedance within about 24 hours of adding effector CTLs at a low E:T ratio (1:1) in cells irradiated with 8 Gy. While irradiation did not appear to significantly enhance SIINFEKL antigen presentation on MHC class I in this model, other potential mechanisms include increased tumor cell immunogenicity and susceptibility to Fas ligand-mediated killing.
Our comparison of single doses of 2 Gy and 8 Gy has clinical relevance for patients with HNSCC. Conventional fractionated IR for HNSCC utilizes 1.8–2.0 Gy doses. While we did not evaluate the effects of multiple treatments with 2 Gy on T-lymphocyte priming or tumor cell sensitivity to CTL killing, mounting evidence suggests that fractionated IR is immunosuppressive[23, 24, 28], potentially due to serial exposure of highly radiosensitive lymphocytes to IR. A comparison dose of 8 Gy was chosen based upon demonstrated safety of stereotactic body radiation therapy in patients with HNSCC with minimal toxicity in the reirradiation setting[29–31]. Our demonstration of enhanced antigen-specific T-lymphocyte priming with higher individual doses of IR will be instrumental for the design of clinical trials utilizing single fraction of hypofractionated IR as an adjuvant for immunotherapy.
Clearly low-dose fractionated IR is the standard of care approach for the upfront treatment of both early and late stage HNSCC. However, this IR regimen is based upon principles of sub-lethal repair of normal cells, redistribution of tumor cells into more radiosensitive portions of the cell cycle, and re-oxygenation of tumor cells and is administered with the intent of inducing direct tumor cell toxicity[28]. In the setting of IR as an adjuvant for immunotherapy, how fractionation and individual fraction doses of IR alter cell death, immunogenicity, antigen release and the function of infiltrating immune cells must be considered. Much work is needed, including determining the effects of single dose versus fractionated radiotherapy on the function of immune cells within the tumor microenvironment and what volume of the tumor needs to be irradiated to have adequate tumor antigen release to induce immunity. Our use of a single cancer model limits the generalizability of these findings, and these results should be validated in other carcinoma models. However, our data demonstrating superior tumor cell antigen release, antigen cross-presentation by APCs, priming of antigen-specific T-lymphocytes and tumor cell sensitivity to CTL killing with a single dose of 8 Gy compared to 2 Gy provides some of the pre-clinical information critical for the appropriate, data-driven design of clinical trials combining IR and checkpoint inhibition.
Research highlights.
Ionizing radiation (IR) induces apoptosis in a dose-dependent fashion
8 Gy IR induces release of tumor antigen to a greater degree than 2 Gy
8 Gy IR induces T-cell priming in vitro to a greater degree than 2 Gy
8 Gy IR induces T-cell priming in peripheral and tumor compartments in vivo
Higher single doses of IR should be considered when combining IR and immunotherapy
Acknowledgments
Financial support: This work was supported by the Intramural Research Program of the NIH, NIDCD, project number ZIA-DC000087 (CTA). RD was supported by through the National Institutes of Health Medical Research Scholars Program, a public-private partnership supported jointly by the NIH and generous contributions to the Foundation for the NIH from Pfizer Inc., The Doris Duke Charitable Foundation, The Newport Foundation, The American Association for Dental Research, The Howard Hughes Medical Institute, and the Colgate-Palmolive Company, as well as other private donors.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Keck MK, Zuo Z, Khattri A, Stricker TP, Brown CD, Imanguli M, et al. Integrative analysis of head and neck cancer identifies two biologically distinct HPV and three non-HPV subtypes. Clin Cancer Res. 2015;21:870–81. doi: 10.1158/1078-0432.CCR-14-2481. [DOI] [PubMed] [Google Scholar]
- 2.Seiwert TY, Burtness B, Mehra R, Weiss J, Berger R, Eder JP, et al. Safety and clinical activity of pembrolizumab for treatment of recurrent or metastatic squamous cell carcinoma of the head and neck (KEYNOTE-012): an open-label, multicentre, phase 1b trial. Lancet Oncol. 2016;17:956–65. doi: 10.1016/S1470-2045(16)30066-3. [DOI] [PubMed] [Google Scholar]
- 3.Topalian SL, Taube JM, Anders RA, Pardoll DM. Mechanism-driven biomarkers to guide immune checkpoint blockade in cancer therapy. Nat Rev Cancer. 2016;16:275–87. doi: 10.1038/nrc.2016.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Taube JM, Klein A, Brahmer JR, Xu H, Pan X, Kim JH, et al. Association of PD-1, PD-1 ligands, and other features of the tumor immune microenvironment with response to anti-PD-1 therapy. Clin Cancer Res. 2014;20:5064–74. doi: 10.1158/1078-0432.CCR-13-3271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Reits EA, Hodge JW, Herberts CA, Groothuis TA, Chakraborty M, Wansley EK, et al. Radiation modulates the peptide repertoire, enhances MHC class I expression, and induces successful antitumor immunotherapy. J Exp Med. 2006;203:1259–71. doi: 10.1084/jem.20052494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gameiro SR, Jammeh ML, Wattenberg MM, Tsang KY, Ferrone S, Hodge JW. Radiation-induced immunogenic modulation of tumor enhances antigen processing and calreticulin exposure, resulting in enhanced T-cell killing. Oncotarget. 2014;5:403–16. doi: 10.18632/oncotarget.1719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gameiro SR, Higgins JP, Dreher MR, Woods DL, Reddy G, Wood BJ, et al. Combination therapy with local radiofrequency ablation and systemic vaccine enhances antitumor immunity and mediates local and distal tumor regression. PLoS One. 2013;8:e70417. doi: 10.1371/journal.pone.0070417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Judd NP, Winkler AE, Murillo-Sauca O, Brotman JJ, Law JH, Lewis JS, Jr, et al. ERK1/2 regulation of CD44 modulates oral cancer aggressiveness. Cancer Res. 2012;72:365–74. doi: 10.1158/0008-5472.CAN-11-1831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Moore E, Clavijo PE, Davis R, Cash H, Van Waes C, Kim Y, et al. Established T Cell-Inflamed Tumors Rejected after Adaptive Resistance Was Reversed by Combination STING Activation and PD-1 Pathway Blockade. Cancer Immunol Res. 2016;4:1061–71. doi: 10.1158/2326-6066.CIR-16-0104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Franken NA, Rodermond HM, Stap J, Haveman J, van Bree C. Clonogenic assay of cells in vitro. Nat Protoc. 2006;1:2315–9. doi: 10.1038/nprot.2006.339. [DOI] [PubMed] [Google Scholar]
- 11.Davis RJ, Silvin C, Allen CT. Avoiding phagocytosis-related artifact in myeloid derived suppressor cell T-lymphocyte suppression assays. J Immunol Methods. 2017;440:12–8. doi: 10.1016/j.jim.2016.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cash H, Shah S, Moore E, Caruso A, Uppaluri R, Van Waes C, et al. mTOR and MEK1/2 inhibition differentially modulate tumor growth and the immune microenvironment in syngeneic models of oral cavity cancer. Oncotarget. 2015;6:36400–17. doi: 10.18632/oncotarget.5063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rahmanian N, Hosseinimehr SJ, Khalaj A. The paradox role of caspase cascade in ionizing radiation therapy. J Biomed Sci. 2016;23:88. doi: 10.1186/s12929-016-0306-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fuertes MB, Kacha AK, Kline J, Woo SR, Kranz DM, Murphy KM, et al. Host type I IFN signals are required for antitumor CD8+ T cell responses through CD8{alpha}+ dendritic cells. J Exp Med. 2011;208:2005–16. doi: 10.1084/jem.20101159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Diamond MS, Kinder M, Matsushita H, Mashayekhi M, Dunn GP, Archambault JM, et al. Type I interferon is selectively required by dendritic cells for immune rejection of tumors. J Exp Med. 2011;208:1989–2003. doi: 10.1084/jem.20101158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gough MJ, Crittenden MR. Combination approaches to immunotherapy: the radiotherapy example. Immunotherapy. 2009;1:1025–37. doi: 10.2217/imt.09.64. [DOI] [PubMed] [Google Scholar]
- 17.Baird JR, Friedman D, Cottam B, Dubensky TW, Jr, Kanne DB, Bambina S, et al. Radiotherapy Combined with Novel STING-Targeting Oligonucleotides Results in Regression of Established Tumors. Cancer Res. 2016;76:50–61. doi: 10.1158/0008-5472.CAN-14-3619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Garnett-Benson C, Hodge JW, Gameiro SR. Combination regimens of radiation therapy and therapeutic cancer vaccines: mechanisms and opportunities. Semin Radiat Oncol. 2015;25:46–53. doi: 10.1016/j.semradonc.2014.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Deng L, Liang H, Burnette B, Beckett M, Darga T, Weichselbaum RR, et al. Irradiation and anti-PD-L1 treatment synergistically promote antitumor immunity in mice. J Clin Invest. 2014;124:687–95. doi: 10.1172/JCI67313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Demaria S, Kawashima N, Yang AM, Devitt ML, Babb JS, Allison JP, et al. Immune-mediated inhibition of metastases after treatment with local radiation and CTLA-4 blockade in a mouse model of breast cancer. Clin Cancer Res. 2005;11:728–34. [PubMed] [Google Scholar]
- 21.Twyman-Saint Victor C, Rech AJ, Maity A, Rengan R, Pauken KE, Stelekati E, et al. Radiation and dual checkpoint blockade activate non-redundant immune mechanisms in cancer. Nature. 2015;520:373–7. doi: 10.1038/nature14292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Scheffer SR, Nave H, Korangy F, Schlote K, Pabst R, Jaffee EM, et al. Apoptotic, but not necrotic, tumor cell vaccines induce a potent immune response in vivo. Int J Cancer. 2003;103:205–11. doi: 10.1002/ijc.10777. [DOI] [PubMed] [Google Scholar]
- 23.Lee Y, Auh SL, Wang Y, Burnette B, Wang Y, Meng Y, et al. Therapeutic effects of ablative radiation on local tumor require CD8+ T cells: changing strategies for cancer treatment. Blood. 2009;114:589–95. doi: 10.1182/blood-2009-02-206870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lugade AA, Moran JP, Gerber SA, Rose RC, Frelinger JG, Lord EM. Local radiation therapy of B16 melanoma tumors increases the generation of tumor antigen-specific effector cells that traffic to the tumor. J Immunol. 2005;174:7516–23. doi: 10.4049/jimmunol.174.12.7516. [DOI] [PubMed] [Google Scholar]
- 25.Debrick JE, Campbell PA, Staerz UD. Macrophages as accessory cells for class I MHC-restricted immune responses. J Immunol. 1991;147:2846–51. [PubMed] [Google Scholar]
- 26.Heit A, Huster KM, Schmitz F, Schiemann M, Busch DH, Wagner H. CpG-DNA aided cross-priming by cross-presenting B cells. J Immunol. 2004;172:1501–7. doi: 10.4049/jimmunol.172.3.1501. [DOI] [PubMed] [Google Scholar]
- 27.Gelbard A, Garnett CT, Abrams SI, Patel V, Gutkind JS, Palena C, et al. Combination chemotherapy and radiation of human squamous cell carcinoma of the head and neck augments CTL-mediated lysis. Clin Cancer Res. 2006;12:1897–905. doi: 10.1158/1078-0432.CCR-05-1761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rosen EM, Fan S, Rockwell S, Goldberg ID. The molecular and cellular basis of radiosensitivity: implications for understanding how normal tissues and tumors respond to therapeutic radiation. Cancer Invest. 1999;17:56–72. [PubMed] [Google Scholar]
- 29.Kress MA, Sen N, Unger KR, Lominska CE, Deeken JF, Davidson BJ, et al. Safety and efficacy of hypofractionated stereotactic body reirradiation in head and neck cancer: Long-term follow-up of a large series. Head Neck. 2015;37:1403–9. doi: 10.1002/hed.23763. [DOI] [PubMed] [Google Scholar]
- 30.Lim CM, Clump DA, Heron DE, Ferris RL. Stereotactic Body Radiotherapy (SBRT) for primary and recurrent head and neck tumors. Oral Oncol. 2013;49:401–6. doi: 10.1016/j.oraloncology.2012.12.009. [DOI] [PubMed] [Google Scholar]
- 31.Siddiqui F, Patel M, Khan M, McLean S, Dragovic J, Jin JY, et al. Stereotactic body radiation therapy for primary, recurrent, and metastatic tumors in the head-and-neck region. Int J Radiat Oncol Biol Phys. 2009;74:1047–53. doi: 10.1016/j.ijrobp.2008.09.022. [DOI] [PubMed] [Google Scholar]